J.ophthalmol.(Ukraine).2017;6:7-10.
|
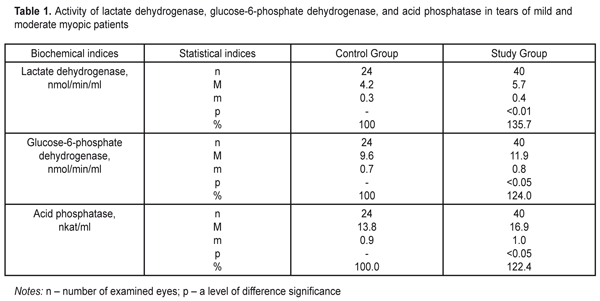
https://doi.org/10.31288/oftalmolzh20176710 Effect of Silicone Hydrogel Contact Lenses on Stability of Cellular and Intracellular Membranes in Corneal Epithelium T.A. Veliksar; N.F. Leus, Dr. Sc. (Med.), Prof.; T.B. Gaydamaka, Dr. Sc. (Med.); I.N. Mikheitseva, Dr. Sc. (Biol.); G.I. Drozhzhyna, Dr. Sc. (Med.), Prof.; S.G. Kolomiichuk, a Research Fellow Filatov Institute of Eye Disease and Tissue Therapy Odessa, Ukraine E-mail: tveliksar@gmail.com Introduction. Contact lenses are increasingly used worldwide for correcting refraction disorders. However, contact lenses can destroy a biochemical composition of the tear. Purpose. To determine the effect of silicone hydrogel contact lenses on the stability of cell membranes and membranes of subcellular structures in the corneal epithelium through detecting marker enzymes in the tear fluid. Material and Methods. We determined the activity of lactate dehydrogenase, glucose-6-phosphate dehydrogenase, and acid phosphatase in tears of patients divided into two groups. Study group comprised 13 people (24 eyes): moderate myopia patients continuously wearing soft contact lenses. Control group consisted of 20 people (40 eyes): moderate myopia patients, spectacles wearers. Results. We revealed a significant increase in the activity of lactate dehydrogenase and glucose-6-phosphate dehydrogenase by >35% and 24%, respectively, as well as a significant increase in the activity of acid phosphatase by 22.4% in the Study group patients comparing to controls. Conclusions. Continuous soft contact lens wearing increases the lability of the corneal epithelium cell membranes. We believe that pharmacological correction of such pathologic changes can prevent the development of severe complications associated with contact lens vision correction. Keywords: cornea, contact lens, lactate dehydrogenase, glucose-6-phosphate dehydrogenase, acid phosphatase Introduction About 120-120 million people worldwide are contact lens (CL) wearers, 87-89 % of them wear soft contact lenses [9, 10]. Once CLs were developed, researchers have made efforts to determine why the cornea becomes more sensitive to infection when using CLs, what happens to the ocular surface and what are the mechanisms of these processes. According to Schein et al., complication development rates for CL wearers in 1989 were 4 persons per 10 000 for daily disposable lenses and 20 persons per 10 000 for continuous wear lenses; and according to Holden et al., those in 2005 were 4.6 per 10 000 and 19.3 per 10 000 for daily disposable lenses and continuous wear lenses, respectively. Thus, despite all developments and improvements in the CL industry, it has not been able to decrease the risk of complication development in contact lens vision correction. There are a lot of CL wear-associated risk factors for keratitis including corneal hypoxia, a state of the tear film, CL wearing schedule and duration, CL cleaning solution ingredients. The oxygen transmissibility of the contact lens is a very important condition but not the only one for “healthy” CL wearing [5]. The abolition of hypoxia does not mean the abolition of CL wear-related inflammatory processes such as red eye syndrome, keratitis, peripheral corneal ulcers or endophthamitis. Contact lenses can destroy a biochemical composition of the tear; they are tightly attached to the corneal surface, thus, excluding, reducing, or, on the contrary, inducing the presence of specific components or provoking the intake of new ones, which leads to lipid peroxidation in tears, denaturation, and proteopexy [11, 12]. The composition of the tear reflexes the condition of eye tissues, particularly of the cornea, and is an indicator for corneal pathologic condition revealing [3, 7, 8, 10]. The literature provides certain evidence of biochemical changes in the tear related to CL wear though it is not enough for clear understanding the mechanisms of these changes. It is known that CL wearing during two hours already causes a shift to anaerobic metabolism in the cornea, which is evidenced by the increased activity of lactate dehydrogenase in tears and the presence of hypoxia-related edema of the cornea as a consequence of enzymatic dysfunction of the endothelial pumps [13]. Contamination, hypoxia, tear production disorders, ineffective lens care solutions and a misuse of CLs play an important role in development of CL wear-associated keratitis. It is necessary to investigate the mechanisms understudied so far, which will make it possible to understand the pathogenesis of CL wear-induced changes in anterior eye tissues. Purpose. To determine the effect of silicone hydrogel contact lenses on the stability of cell membranes and membranes of subcellular structures in the corneal epithelium through detecting marker enzymes in the tear fluid. Material and Methods Thirty-three patients (64 eyes) with moderate myopia were involved in the study. Study group comprised 13 people (24 eyes): moderate myopia patients continuously wearing soft contact lenses. Control group consisted of 20 people (40 eyes): moderate myopia patients, spectacles wearers. Among 13 Study group patients there were 10 (76.9%) women and 3 (23.1%) men. The age averaged 27.45±1.43 between 17 and 49. The mean CL wear duration was 93.76 ± 13.51 months. The mean values for uncorrected visual acuity and best-corrected visual acuity were 0.122 ±0.020 and 0.845±0.026, respectively. All the patients made little subjective complaints if any and applied for soft contact lens replacement. All examined patients wore non-ionic low-hydrophilic soft contact lenses (up to 50% of water) referring to the first group according to Food and Drug Administration (FDA) classification. For 20 patients of Control group, a women/men ratio was 13 (65%) / 7 (35%). The age averaged 27.69±2 and ranged from 20 to 48. The mean values for uncorrected visual acuity and best-corrected visual acuity were 0.265 ± 0.139 and 0.963±0.197, respectively. We determined the activity of lactate dehydrogenase, glucose-6-phosphate dehydrogenase, and acid phosphatase [2]. The lactate dehydrogenase activity was detected using H. Bergmeyer’s method based on assessing a rate of enzymatic oxidation of reduced nicotin-amide-adenine-dinucleotide when lactate is formed from pyruvate. The rate was spectrophotometrically registered as an increase in optical density of the studied solution at 340 nm [2]. The glucose-6-phosphate dehydrogenase activity was detected using G. Lohr’s method. The method is based on changes in reduction rates of nicotinamide adenosine dinucleotide phosphate (NADF) in incubation medium with saturating substrate concentrations, cofactors, and the optimal pH value at 340 nm [2]. The acid phosphatase activity was detected using a micro method which is based on assessing the concentration of para-Nitrophenylphosphate, a free organic substrate, which is formed as enzyme reaction at 410 nm [2]. The changes in the optical density of the studied solutions were measured using a Specol–210 spectophotometer in a 1 cm cuvette. The data obtained were processed by SPSS 11.0 software [1]. Results and Discussion The lactate dehydrogenase activity level was increased to 5.7±0.4 nmol/min/ml in the Study group, which comprised 135.7% in relation to the Control group where the lactate dehydrogenase activity level was 4.2±0.3 nmol/min/ml (Table 1).
The values of the glucose-6-phosphate dehydrogenase activity were increased in the Study group comprising 124% in relation to the Control group, i.e. 11.9±0.8 nmol/min/ml vs. 9.6 ± 0.7 nmol/min/ml, respectively (Table 1). The level of acid phosphatase activity in tears was increased in the Study group equaling 122.4% as compared to the Control Group, i.e 16.9±1.0 nkat/ml vs. 13.8±0.9 nkat/ml, respectively. Analyzed the activity of reductive-oxidative enzymes in tears of continuous soft lens wearers, we revealed a statistically significant increase in the activity of lactate dehydrogenase and glucose-6-phosphate dehydrogenase, by 35% and 24%, respectively. The normal values for the activity of the pointed enzymes in tears reflexes a degree of corneal epithelium destruction since they are localized in the cytoplasm of the epithelial cells. Elevated activity of these enzymes in tears gives evidence of intensive corneal cell disruption caused by contact lens wearing. Under the same conditions, we revealed a significant increase, by 22.4%, in the activity of lysosomal marker enzymes of acid phosphatase in tears of patients with continuous wear soft contact lenses. This fact also confirms the previous statement that contact lens visual correction induces the destructive processes in cellular and intracellular structures of the epithelial cells. Pathogenesis of chemical changes, which we revealed in tears under contact lens vision correction conditions, comes from the increased lability of epithelial cell membranes and subcellular organelles. This process can be based on insufficient oxygen supply to corneal tissues and, as a result, formation of insufficiently oxidized metabolic products such as aldehydes, acids etc. Such conditions are known to stimulate lipid peroxidation, which can lead to membrane lipid component damage. The data obtained reveal an important part of pathogenic effect of contact lenses on the cornea as well as extent and complete our vision of the state of the cornea when wearing contact lenses. Conclusions Firstly, continuous soft contact lens wearing increases the lability of corneal epithelial cell membranes, which is evidenced by a statistically significant increase in intracellular reductive-oxidative enzyme activity in tears: by 35.7% and 24% for lactate dehydrogenase and glucose-6-phosphate dehydrogenase, respectively. Secondly, a significant increase in the lability of intracellular membranes of the corneal epithelium was noted, which is pointed out by elevated activity (by 22.4%) of acid phosphatase lysosomal enzymes in tears. Reference
|