J.ophthalmol.(Ukraine).2018;3:52-56.
|
https://doi.org/10.31288/oftalmolzh201835256 Activity of plasminogen activator inhibitor-1 in blood of patients with metabolic syndrome depending on a stage of diabetic retinopathy Serdiuk V. N.,1 Dr Sc (Med); Kyryliuk M. L.,2 Dr Sc (Med), Prof.; Pylypenko L.Yu.,3 Post-grad Student, Ophthalmologist 1 Dnipropetrovsk Medical Academy, Health Ministry of Ukraine; Dnipropetrovsk Regional Clinical Ophthalmology Hospital; Dnipro (Ukraine) 2 Ukrainian Research Center for Endocrine Surgery, Transplantation of Endocrine Organs and Tissues, Health Ministry of Ukraine; Kyiv (Ukraine) 3 Dnipropetrovsk City Policlinics No4; Dnipro (Ukraine) E-mail: kzdokol@ukr.net, kmlazar@ukr.net, l-u-shevchenko@yandex.ua TO CITE THIS ARTICLE: Serdiuk VN, Kyryliuk ML, Pylypenko LYu. Activity of plasminogen activator inhibitor-1 in blood of patients with metabolic syndrome depending on a stage of diabetic retinopathy. J.ophthalmol.(Ukraine).2018;3:52-56. https://doi.org/10.31288/oftalmolzh201835256 Background. The main cause for the development of proliferative diabetic retinopathy (DRP) is the absence of capillary perfusion, resulting in the initiation of retinal neovascularization. In ischemia reperfusion injury of the retina, the plasminogen activator inhibitor 1 (PAI-1), inflammatory mediators, cytokines, adhesion factors activated in the metabolic syndrome (MS) may be involved. Therefore, there is a necessity to evaluate the role of PAI-1 in the initiation and progression of DRP in MS. Purpose. To study the activity of PAI-1 in blood of patients with the metabolic syndrome (MS) depending on a stage of diabetic retinopathy (DRP) and in relation with a level of circulating interleukin-8 (IL-8) and blood fibrinogen. Material and Methods. Studies were carried out in 64 patients (95 eyes) with MS and DRP, comparable in sex, age and duration of type 2 diabetes mellitus (T2DM), which were divided into 3 groups depending on a stage of DRP. The control group consisted of 23 individuals of both sexes with obesity without T2DM. The activity of PAI-1 and the concentration of circulating IL-8 in blood were determined by enzyme immunoassay; fibrinogen was assessed by the clotting method. Statistical analysis included single-factor analysis of variance (ANOVA) and regression analysis. Results. It was shown that only in the case of conditional combination of DRP stages II and III in one stage, the activity of PAI-1 was statistically significantly lower in comparison with the control group ((21.3 ± 2.8 U / ml (95% CI 17.3-25, 3) vs 12.0 ± 3.3 U / ml (95% CI 7.43-16.6), p < 0.05)), which did not occur in DRP stage (p> 0.05). In the group of patients with T2DM, regardless a stage of DRP, the level of PAI-1 activity was positively associated with circulating IL-8 concentration (r = 0.43, R2 = 18.6 %, N = 54, p = 0.001). Also, an inverse linear dependence of blood fibrinogen concentration on the PAI-1 activity was revealed (r = -0.36, R2 = 13.6%, N = 55, p = 0.005). Conclusion. Patients with DRP and MS had significantly decreased PAI-1 at DRP stages II and III compared to non-diabetic obese patients (р<0.05) unlike the patients with DPR stage I (p>0.05); a strong significant positive association between PAI-1 and circulating IL-8 (p=0.001) and a significant negative linear relationship between blood fibrinogen concentration and PAI-1 (p=0.005) were revealed. Keywords: diabetic retinopathy, metabolic syndrome, plasminogen activator inhibitor-1
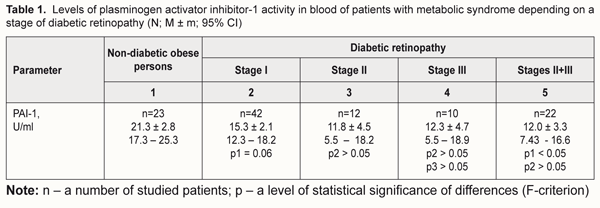
Introduction A major cause of proliferative diabetic retinopathy (DRP) is the absence of capillary perfusion, resulting in the retinal neovascularization onset [10]. A key role in capillary occlusion disorders belongs to activation of various adhesion molecules including vascular сell аdhesion molecule (1VCAM-1), inter-cellular adhesion molecule 1 (ICAM-1) and others. Nevertheless, leukocyte adhesion is not the only cause of leukostasis and capillary blood flow disorders in diabetes mellitus (DM). There are other DM-correlating concomitant mechanisms and factors for retinal damage, among which platelet aggregation is of importance [16]. One of the local regulators of the fibrinolytic system in blood is plasminogen activator inhibitor-1 (PAI-1). The gene encoding human PAI-1 was cloned from a λEMBL3 genomic library and was found to span approximately 12 kb and to contain eight introns [9]. PAI-1 is referred to a group of serine protease inhibitors and also called Serpine-1. PAI-1 is mainly secreted by adipocytes, endothelial cells, and hepatocytes and deposited in platelets in a latent form. PAI-1 serves as a main negative regulator of fibrinolysis due to its role as a primary inhibitor of tissue plasminogen activator (tPA). PAI-1 is the major antagonist to tPA and urokinase plasminogen activator (uPA) which activate fibrinolysis-inducing plasminogen. Earlier studies have reported on associations of the elevated PAI-1 levels with obesity [4, 8], insulin resistance, impaired glucose tolerance [8, 14], and type 2 DM (T2DM) [5, 15]. In the context of modern science, a role of PAI-1 in initiation and progression of DRP needs to be assessed [19]. PAI-1 and other inflammatory mediators activated in the metabolic syndrome such as ICAM-1, interleukin-1 (IL-1) as well as coagulation factor III and vascular endothelial growth factor (VEGF) can induce ischemia reperfusion injury of the retina and retinal neovascularization [18]. Interaction between PAI-1 and transforming growth factor-β (TGF- β), in the end, can also induce neovascularization, which is observed in retinopathy in the presence of T2DM, as a consequence of a VEGF action [13]. So, it is deemed quite believable that this blood-circulating protein can be a predictor for the beginning of proliferation in DRP, which gains grounds to further studies in this direction. Purpose. To study the activity of PAI-1 in blood of patients with the metabolic syndrome (MS) depending on a stage of diabetic retinopathy (DRP) and in relation with a level of circulating interleukin-8 (IL-8) and blood fibrinogen. Material and Methods The study involved 64 patients (95 eyes) with MS and DRP (men and women; age, 61.55±2.37 years; DM duration, 11.23±2.11 years; a glycated hemoglobin (HbA1C) level, 9.89±0.78 %; body mass index (BMI), 34.55±3.75 kg/m2) divided into 3 groups based on a DRP stage. Control group included 26 non-diabetic obese persons (men and women; age, 62.47±4.73 years; body BMI, 31.87±3.92 kg/m2). The study followed the requirements of Declaration of Helsinki, Orders No 281 dated 1st November 2000, No 355 dated 25th September 2002, and No1118 dated 21st December 2012 of Ministry of Health of Ukraine. Patient enrollment followed the recommendations previously described in the literature [3]. MS was diagnosed according to Adult Treatment Panel III (ATP III) criteria [12, 17]. We used a glucose oxidase test to determine glucose levels in blood plasma; high-pressure liquid chromatography to assess HbA1C levels in blood; a spectrophotometric method to assess levels of triglycerides, total cholesterol and cholesterol fractions; a clotting test to assess blood fibrinogen levels. IL-8 concentration in blood was determined using an enzyme-linked immunosorbent assay (ELISA) by a Human Interleukin-8 ELISA®kit; ELISA by a PAI-1Antibind®ELISA kit was used to assess the PAI-1 activity in blood. Half lifetime of an active PAI-1 molecule in the blood flow is approx. 2 hours. This is why, likely, there are no clear reference values of the PAI-1 activity. According to the kit used in the study, the PAI-1 activity in blood can be determined within the reference values of 0-40 U/ml. In the literature, there are control values of PAI-1 for persons with no signs of autoimmune diseases and thrombosis at examination, during the follow-up period, and in history (15.8±0.92 U/ml) [1]. All patients were performed a comprehensive ophthalmic examination including autorefractometry, visual acuity testing, tonometry, perimetry, biomicroscopy, eye fundus imaging, and fluorescent angiography (if indicated). DRP was diagnosed according to the Order of Ministry of Health of Ukraine No356 dated 22nd May 2009, edited in Order No 574 dated 5th August 2009, which recommends determining non-proliferative, pre-proliferative, and proliferative stages of DRP. Pre-proliferative and proliferative stages were conditionally combined in one stage in order to optimize the obtained data when assessing impact factors in T2DM progression. Statistical processing was performed using a one-way ANOVA test and regression analysis. Regression model characteristics were considered as follows: r, correlation coefficient; R2, determination coefficient; p, a level of statistical significance of differences. Statistical characteristics are given as mean (M) and standard error (± m), 95% confidence interval (95% CI). Differences were accepted statistically significant with р<0.05. SPSS 9.0 software was used for statistical data analysis. A Statgraphics 3 software package for Windows was used to calculate and design graphs in regression analysis. Results Studying the activity of PAI-1 as a possible diagnostic criterion for DRP development revealed a number of relationships. Findings of the statistical analysis showed that mean values of the PAI-1 activity in blood in all groups exceeded the control values described in the literature [1]. A gradual but insignificant (p>0.05) decrease in the PAI-1 activity was noted in the patients with T2DM and various DRP stages compared to the patients with the metabolic syndrome (Table 1).
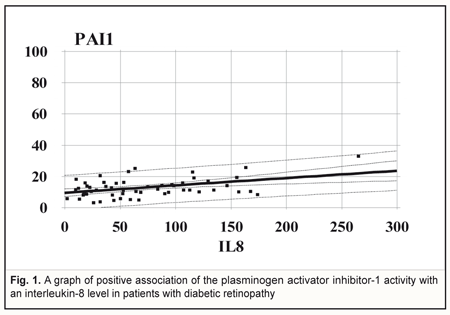
Comparing the PAI-1 activity between the non-diabetic obese patients and diabetic patients with stage I DRP revealed a tendency to the decreased PAI-1 activity in stage I DRP (р=0.06). However, when stages II and III were combined into one stage of DRP, the PAI-1 activity became significantly lower (р<0.05) compared to controls only. In the group of T2DM (n=54), regardless a DRP stage, the PAI-1 activity level was positively associated with the level of circulating Il-8. A graph of association is given as a line with parameters: r = 0.43; R2 = 18.6%; p=0.001 (Fig. 1).
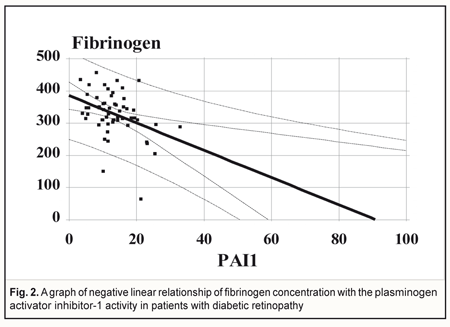
When performing regression analysis, with fibrinogen concentration in blood taken as a dependent variable (Y) and a PAI-1 value taken as an independent variable (X), there is a linear dependence of fibrinogen concentration on the PAI-1 activity in the DRP patients (n=55) with parameters: r = -0.36; R2 = 13,6%; p=0.005 (Fig. 2).
Discussion Since data in the literature are heterogeneous and PAI-1 and T2DM association assessment is required [11], 52 epidemiological studies, including databases of EMBASE, PubMed, Web of Science, and Cochrane Library, within the period to 2015 have been analyzed. Of 47 comparisons (cross-sectional), 34 (72%) revealed significantly elevated PAI-1 among diabetes cases versus controls, 2 (4%) reported significantly elevated PAI-1 among controls, and 11(24%) reported null effects. Therefore, findings from this systematic review confirm a link between PAI-1 and T2DM, independent of established diabetes risk factors [19]. That is why scientists’ attention is drawn to studies of MS in relationship with DRP and the PAI-1 activity. It is clear that the activity of the anticoagulant (fibrinolytic) system is reduced if PAI-1 is elevated in blood, which leads to a risk of thrombosis. In our study, with DRP stages II and III conditionally combined into a single one, the PAI-1 activity was found statistically lower (р<0.05) compared to controls (non-diabetic obese people) due to a narrowed corridor of confidence interval with almost equal values at all stages, which was not when comparing to DRP stage I (p>0.05). So, we showed that PAI-1 expression occurs in all DRP stages combined with obesity, which is in agreement with a concept of the decreased activity of the anticoagulant (fibrinolytic) system in patients with the metabolic syndrome. Elevated PAI-1 in the metabolic syndrome is, likely, a kind of foundation for endothelial adhesion dysfunction and neovascularization in T2DM. A high PAI-1 level in obesity can be explained by the fact that people with the metabolic syndrome received no antiplatelet therapy unlike T2DM patients who are at high risk of cardiovascular events. It is likely that elevated PAI-1 in early DRP (high risk of thrombosis and capillary occlusion) is of certain significance for proliferative processes in the retina. Data in the literature have demonstrated an upregulation of uPA and its receptor uPAR in the retina of animals with early DRP and retinal neovascularization. PAI-1 expression has also been studied in an experimental model of early DRP and retinal neovascularization. The PAI–1 expression is significantly increased in mice with developing retinal neovascularization. In the retina of experimental mice, PAI–1 mRNA is almost 12 fold increased at the beginning of neovascularization and the PAI-1 level is 5 fold higher compared to control in the period of active angiogenesis. At the end of the angiogenic period, prior to the beginning of vascular regression, the PAI–1 mRNA level decreases significantly in the experimental model (1.6 times fold than controls). A similar increase in PAI–1 mRNA (5.74 times higher than control) is seen in the retina of diabetic animals which have increased vascular permeability. Authors have concluded that the components of the uPA, uPAR, PAI–1 system are upregulated in both early diabetes and active retinal neovascularization. Increased cell motility is a characteristic of the angiogenic process and high levels of PAI–1 are associated with increased cell motility as a result of the cell/matrix interaction regulation. High levels of PAI–1 in the retina in DM can play a certain role in creating an environment which contributes to formation of new vessels at the later stages of diabetes [7]. Thus, PAI-1 can be an important target molecule for pharmacological intervention in DRP. In our study, pair-wise regression analysis to determine a correlation relationship between PAI-1, IL-8, and fibrinogen showed a metabolic concordance of endothelial function abnormalities in the patients with DRP and MS. Thus, independent on a DRP stage, a PAI-1 level was significantly (p=0.001) positively associated with a level of IL-8, i.e. PAI-1 elevation was proportional to the growth of IL-8 in the whole interval of individual values, which supports the idea that cytokines in peripheral blood may be predictors for proliferative DRP. In that way, diabetes in family history, elevated PAI-1 levels in blood, smoking, and diabetes duration have been found to be four positive predictors associated with proliferative DRP [20]. The Veterans Affairs Diabetes Trial (VADT) has revealed a relationship not only between plasma fibrinogen concentration and a type of antihyperglycemic therapy, but also the fact that a baseline PAI-1 level is an independent risk factor for the onset of DRP which significantly increases by 12% for each 10 ng/dL increase in baseline PAI-1 concentration (odds ratio [OR] 1.012 [95% CI 1.00–1.024], P = 0.042) [6]. On the assumption of our data, a relationship between fibrinogen and PAI-1 is more significant at later DRP stages. Conclusion Patients with DRP and MS had significantly decreased PAI-1 at DRP stages II and III compared to non-diabetic obese patients (р<0.05), which was not when comparing to the patients with DPR stage I (p>0.05); strong significant positive association between PAI-1 and circulating IL-8 (p=0.001) and significant negative linear dependence of blood fibrinogen concentration and PAI-1 (p=0.005) were revealed. References 1.Aisina RB, Mukhametova LI, Ostryakova EV et al. [Polymorphism of the plasminogen activator inhibitor type 1 gene, plasminogen level and thromboses in patients with the antiphospholipid syndrome]. Biochem. Moscow Suppl. Ser. B. 2013; 7(1):1-15. In Russian. 2.Pasechnikova NV, Metelicyna IP, Naumenko VA, Beliaev VD. [Change in the level of some pro- and anti-inflammatory cytokines in patients with diabetic retinopathy after laser retinal coagulation]. Oftalmol Zh. 2010;2:4-7. In Russian. 3.Serdyuk VN, Ishhenko VA. [Morphometrial and biochemical clusters of metabolic syndrome in patients with type 2 diabetes mellitus at different stages of diabetic retinopathy]. Mizhnarodnyi endokrynolohichnyi zhurnal. 2016;7(79):69-74. In Russian. 4.Alessi MC et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–7. 5.Al-Hamodi, Z, et al. Association of plasminogen activator inhibitor-1 and tissue plasminogen activator with type 2 diabetes and metabolic syndrome in Malaysian subjects. Cardiovasc Diabetol. 2011;10:23. 6.Azad N, et al. VADT Study Group. Association of PAI-1 and fibrinogen with diabetic retinopathy in the Veterans Affairs Diabetes Trial (VADT). J Clin Diagn Res. 2015 Jan;9(1):BC18-21. doi: 10.7860/JCDR/2015/10712.5449. 7.Das A, et al. Plasminogen Activator Inhibitor–1(PAI–1) in Early Diabetic Retinopathy and Retinal Neovascularization. Investigative Ophthalmology & Visual Science. 2005;46(13):2367. 8.Festa A. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. The Insulin Resistance Atherosclerosis Study (IRAS). Arterioscler Thromb Vasc Biol. 1999;19:562–8. 9.Follo M, Ginsburg D. “Structure and expression of the human gene encoding plasminogen activator inhibitor, PAI-1,” Gene, vol. 84, no. 2, pp. 447–453, 1989. 10.Garner A. Histopathology of diabetic retinopathy in man. Eye. 1993;7(2):250–3. 11.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–72. 12.Grundy SM, et al. Diagnosis and Management of the Metabolic Syndrome. Circulation. 2005;112:2735–52. 13.Liao H, Hyman MC, Lawrence DA, Pinsky DJ. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1α, and C/EBPα. FASEB Journal. 2007;21(3):935–49. 14.Meigs JB, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: The Framingham Offspring Study. JAMA. 2000;283:221–8. 15.Nagi DK, et al. Plasminogen Activator inhibitor (PAI-1) activity is elevated in asian and caucasian subjects with non-insulin-dependent (Type 2) Diabetes but not in those with impaired glucose tolerance (IGT) or non-diabetic Asians. Diabet Med. 1996;13:59–64. 16.Sagel J, et al. Increased platelet aggregation in early diabetes mellitus. Ann Intern Med. 1975;82:733–8. 17.Scott MG, et al. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-97. 18.Yan F, Pinsky DJ, Mackman N, Stern DM. Egr-1: is it always immediate and early? Journal of Clinical Investigation. 2000;105(5):553–4. 19.Yarmolinsky J, et al. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. 2016; 6:17714. doi: 10.1038/srep17714. 20.Zhong Ze-Long, Chen Song. Plasma Plasminogen Activator Inhibitor-1 Is Associated with End-Stage Proliferative Diabetic Retinopathy in the Northern Chinese Han Population. Experimental Diabetes Research 2012;2012:Article ID 350852.
|