J.ophthalmol.(Ukraine).2019;1:9-16.
|
http://doi.org/10.31288/oftalmolzh20191916 Received: 14 October 2018; Published on-line: 28 February 2019 Cranial nerve III and/or VI function abnormalities associated with active herpesvirus infection: Clinical experience and literature review I.G. Vasilyeva, Cand Sc (Biol); V.N. Zhdanova, Cand Sc (Med); N.G. Chopik, Cand Sc (Biol); T.A. Makarova, Jun Res Fellow; E.S. Galanta, Res Fellow; O.I. Tsiubko, Res Fellow Romodanov Neurosurgery Institute, Kyiv (Ukraine) E-mail: vigvasileva@gmail.com TO CITE THIS ARTICLE: Vasilyeva IG, Zhdanova VN, Chopik NG, Makarova TA, Galanta ES, Tsiubko OI.Cranial nerve III and/or VI function abnormalities associated with active herpesvirus infection: Clinical experience and literature review. J.ophthalmol.(Ukraine).2019;1:9-16.http://doi.org/10.31288/oftalmolzh20191916 Background: The field of oculomotor abnormalities (OMA) is of clinical and social importance. There is a group of OMA of unknown etiology. Purpose: To investigate the presence of DNA of herpesviruses venous blood of patients with OMA resulting from lesions of cranial nerves (CN) III and/or VI. Materials and Methods: Thirty-five patients with OMA of unknown etiology underwent examination. Whole venous blood samples were collected for detection of herpes simplex virus type 1/2 (HSV1/2), cytomegalovirus (CMV), Epstein Barr virus (EBV), human herpes virus 6 (HHV6), HHV7, varicella-zoster virus (VZV), and HHV8 DNA using polymerase chain reaction. Results: HHV7 was most common (16 patients; 45.7%), followed by EBV (5; 14.3%), HHV6 (2; 5.7%), HSV1/2 (1 patient) and CMV (1 patient). Eight patients (22.8%) were found to have two viruses. Of these, 6 had both EBV and HHV7, one had both EBV and HHV6, and one had both HHV6 and HHV7. In addition, 2 patients (5.7%) were found to have three viruses, EBV, HHV6, and HHV7. Conclusion: For patients with OMA of unknown etiology, the venous blood should be tested by PCR for herpesviruses. Keywords: oculomotor abnormalities, herpesviruses
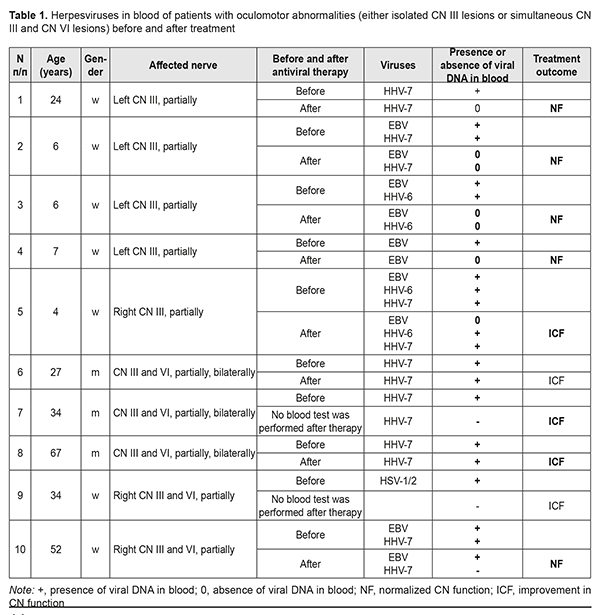
Introduction Oculomotor abnormalities (OMA) are most common in young adults of working age. In Ukraine, approximately 50 thousand children are newly diagnosed with strabismus every year. The prevalence of strabismus in children has been reported to vary from 0.5% to 3.5% across studies [1, 2]. Neuroimaging methods, brain computed tomography (CT) and magnetic resonance imaging (MRI) enabled clinicians to improve the diagnosis of central nervous system (CNS) diseases, including OMA. There is, however, a group of OMA of unknown etiology, although its share declined from 30% in nineteen sixties to 10-20% in nineteen seventies-eighties and 4-6% in nineteen nineties [3]. It is believed that a portion of cases with OMA of unknown etiology is caused by viral infections of cranial nerves (CN). The introduction of nucleic acid amplification technologies in ophthalmology made it possible to demonstrate that infection with herpes viruses is a common cause of these diseases [4, 5]. There have been reports on the molecular mechanisms of inoculation, dissemination, persistence, latency and reactivation of viruses in inflammatory processes in the ocular tissue. It has been demonstrated that human herpes virus 6 (HHV6) and other herpes viruses may cause inflammatory processes, uveitis and endophthalmitis [6]. Gonsalez-Gonsalez et al [7] found that herpes viruses accounted for 7% of all scleritis cases. Herpesviruses are transported along cranial nerves (CN). After inoculation of the cornea, viral antigen was found in ocular nerves [8]. One to three days later antigen was also found in the iris, ciliary body and choroid/sclera [9] suggesting that virus spread to these tissues occurred via their nerve supply [8]. After inoculation of the snout, virus was isolated from ophthalmic and maxillary parts of the trigeminal ganglion and the superior cervical ganglion and then from the brainstem, eye and mandibular part of the trigeminal ganglion [8]. In the hamster infection model [10], at 96 hours after conjunctival inoculation with EHV-9, encephalitis was observed in the brainstem at the level of the pons and cerebellum. The results suggested that EHV-9 invaded the brain via the trigeminal nerve in addition to the abducent (CN VI), oculomotor (CN III), and facial nerves. A feature of herpesviruses is their ability to remain latent in the body lifelong. Trigeminal ganglia are known as sites of latent infection by herpes [11]. In remission, only latency-associated transcripts were detected in the ocular tissues [11]. Although the mechanisms of reactivation of the viruses have not been identified, the presence of immunosuppressive state is believed to be an important precondition for viral reactivation [11, 12]. Replication in the cornea of reactivated virus from trigeminal ganglia produces epithelial lesions, with inflammation due to attraction of immune cells [13]. Cranial nerves are not only the pathways along which viruses are transported from one tissue to another, but are also sites of pathological changes resulting in their dysfunction [8, 14-17]. The mechanisms of viral injury to CN cells have not been fully elucidated yet; there have been, however, case reports confirming the association between cranial nerve injury and herpetic infection, with an adequate antiviral therapy resulting in improvement or complete restoration of CN function [15-17]. In spite of some advances in understanding the mechanisms of ocular infection with herpes viruses, there are only isolated studies addressing the investigation of virus-related oculomotor abnormalities. Most studies address the investigation of virus-induced inflammatory response in the ocular tissue. The purpose of the study was (1) to investigate the presence of DNA of herpes simplex virus (HSV)1/2, cytomegalovirus (CMV), Epstein Barr virus (EBV), varicella-zoster virus (VZV), human herpes virus (HHV)6, HHV7, and HHV8 in venous blood of patients with OMA related to lesions of CN III and/or VI, and (2) to generalize the data from the studies addressing the investigation of tropicity of herpes viruses and their cytopathic effect on nerve tissue. Materials and Methods Medical records of 35 patients that visited the Romodanov Neurosurgery Institute for OMA during 2016 to 2018 were subjected to analysis. Patients underwent clinical-and- neurological, and neuro-ophthalmological examination and neuroimaging studies (brain MRI or CT). No neurosurgical disorder was found in any patient. Inclusion criteria were the presence of CN III and/or CN VI lesions, and absence of neurosurgical or endocrine disorders or autoimmune processes. Whole venous blood samples were collected for detection of HSV1/2, CMV, EBV, HHV6, HHV7, VZV, and HHV8 DNA using polymerase chain reaction (PCR). An Ampliprime DNK-sorb kit was used to isolate DNA from blood samples. A classic PCR was performed using a GenPak PCR-core kit (Isogene, Russia). The reagents used were agarose (Amresco, Solon, OH, USA), Trilon B (Riedel-de Haën AG, Seelze, Germany), Tris (Amresco), and ethidium bromide (Sigma). PCR amplification was performed in a Tertsik PCR system (DNK Tekhnologiya), and 2% agarose gel electrophoresis and ViTran software were used to visualize PCR amplification products. Fischer’s exact test was used to assess the significance of the difference in sign frequency among comparison groups. The study protocol was approved by the Ethics Committee of the Romodanov Neurosurgery Institute. Results and Discussion The most common presenting complaint was diplopia (33 patients; 94.3%). In addition, patients complained of cosmetic concerns with regards to convergent strabismus (23 patients; 65.7%), divergent strabismus (8 patients; 22.95%), and ptosis (6 patients; 17.1%). Two (5.7 %) patients had ptosis without diplopia. The study cohort included 20 (57.14%) women and 15 (42.86%) men. The patients’ age ranged from 4 to 67 years (mean age, 31.2±14.4 years), i.e., the bulk of patients were adults of working age. In addition, there were 9 (25.7%) children aged 4 to 14 years. Oculomotor abnormalities were caused by unilateral (30 patients; 85.7%) or bilateral (5 patients; 14.3%) abnormalities in the function of CN III and/or CN VI. Isolated CN III lesion was found in 5 patients (2 cases of right CN III lesion and 3 cases of left CN III lesion). All the five had a partial loss of CN III function. Of these, 2 children had a superior rectus paresis, one had an inferior rectus paresis, and one had an isolated second-degree ptosis. A woman of 24 years had a left second-degree ptosis and partial inferior rectus paresis. It is noteworthy that four (80%) of these five were children (girls) of 4 to 7 years. Another 5 patients had simultaneous lesions of CN III and VI (3 men with bilateral lesions and 2 women with right simultaneous partial lesions of CN III and VI). Herpesviruses were found in all the 10 patients with isolated CN III lesions or simultaneous lesions of CN III and VI. Of these, 4, 1, 2, 1 and 1 had HHV7 only; EBV only; HHV7 and EBV; HHV6 and EBV; HHV6, HHV7, and EBV; and HSV1/2, respectively (Table 1).
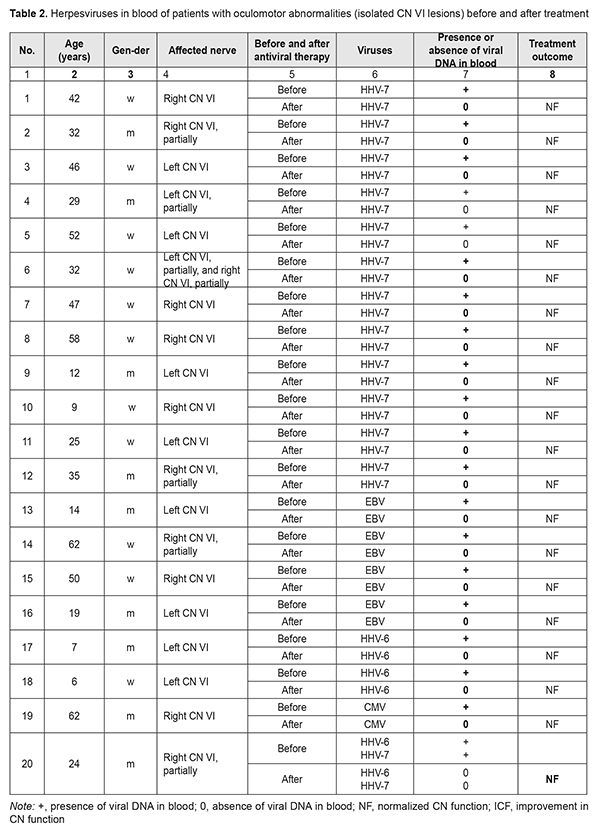
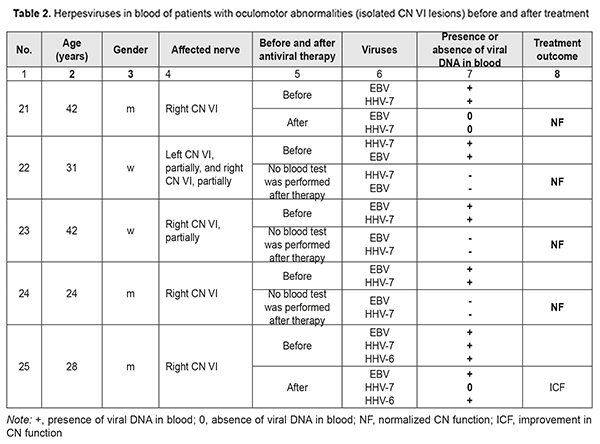
Isolated abnormalities in the function of CN VI were found in 25 patients (13 and 10 had right-side and left-side abnormalities, respectively, and 2 had partial bilateral neuropathy). In these patients, abnormalities varied from paralytic convergent strabismus and absence of ocular motility to partial ocular motility defect (restricted lateral gaze). In addition, of these patients, 3 were children (girls aged 6 and 9 years and a boy aged 12 years), and they had complete CN VI dysfunction. Of the patients with abnormalities in the function of CN VI, 12, 2, 4, 1, 4, 1, and 1 had HHV7 only; HHV6 only; EBV only; CMV only; HHV7 and EBV; HHV6 and HHV7; and HHV7, HHV6, and EBV, respectively (Table 2).
The identification of etiology of CN dysfunction facilitated the selection of the strategy for further treatment of patients and provided for the improvement in their quality of life. All patients showed partial or complete resolution of OMA after etiotropic treatment. Twenty-nine (82.86%) patients had complete resolution of functions of affected cranial nerves. Of these, 25 (86.2%) were found to have no herpesviruses in their blood, and 4 did not undergo blood tests for herpesviruses after treatment (Tables 1 and 2). Six patients showed partial resolution of OMA after treatment. Of these, 3 men (of 27, 34 and 67 years) had simultaneous bilateral lesions of CN III and VI at baseline, and were found to have HHV7 in their blood both before and after treatment. In addition, a 34-year-old woman with pre-treatment simultaneous lesions of CN III and VI associated with the presence of HSV1/2 in her blood did not undergo blood tests for herpes viruses after treatment. Moreover, after treatment, a 4-year-old girl with a pre-treatment right partial CN III lesion (superior rectus paresis) caused by EBV, HHV6, and HHV7, (1) showed an improvement in CN III function abnormality, with improvements in vertical strabismus, loss of upward gaze, and the degree of diplopia, and (2) was found to have HHV6 and HHV7, but not ЕВV in the venous blood (Table 1). Furthermore, after treatment, a 28-year-old man with a pre-treatment right CN VI lesion associated with the presence of EBV, HHV6, and HHV7 in his venous blood, (1) showed an improvement in loss of CN VI function, and (2) was found to have HHV6 and HHV7, but not ЕВV in his venous blood (Table 1). The duration of OMA in these 6 patients exceeded 5 years (Table 2). In venous blood samples of patients with OMA related to simultaneous lesions of CN III and VI, HHV7 was more common compared to HHV6, EBV, HSV1/2, and CMV (Р2t = 0.49*10–4; Р2t = 0.95*10–2; Р2t = 0.59*10–7; and Р2t = 0.59*10–7, respectively) (Tables 1, 2). There was no significant difference in incidence of the presence of HHV7 in venous blood between patients with OMA related to isolated lesions of CN III and those with OMA related to simultaneous lesions of CN III and VI (Р2t =0.58) or those with OMA related to isolated lesions of CN VI (Р2t =0.62). The same was true with regard to incidence of the presence of HHV6 (Р2t = 0.22, Р2t = 0.29, respectively) or EBV (Р2t =0.30, Р2t =0.36, respectively). There was no significant difference in incidence of co-infections with several herpesviruses between patients with OMA related to isolated lesions of CN III and those with OMA related to simultaneous lesions of CN III and VI (Р2t =0.29) or those with OMA related to isolated lesions of CN VI (Р2t =0.14) (Tables 1 and 2). Biological characteristics of beta- herpesviruses HHV7 and HHV6, gamma-herpesvirus EBV and the capacity of these viruses to infect cranial nerves HHV7 and HHV6 which were found in the venous blood of patients with OMA belong to the betaherpesvirus subfamily. It is believed that 90% of children infected with these viruses become infected during the first three years of life. Primary infection occurs through the oral cavity, where the virus replicates in the salivary glands, tonsils or cervical lymph nodes [18, 19]. The growth cycle lasts several days and is followed by a lifelong persistent infection; these tissues act as a depot for the storage of viruses [20, 21]. HHV7 and HHV6 can disseminate through the body during primary infection or reactivation under conditions of poor immune control. Systemic dissemination occurs via bloodstream and along lymphatic vessels through the interaction of mononuclear cells of peripheral blood and adhesion of these cells to endothelial cells. HHV7 and HHV6 enter the central nervous system through the olfactory pathway without crossing the blood-brain barrier [14]. This may result in the development of secondary infection, acute or latent, in various organs [9, 22-29]. Cases of acute HHV7 encephalitis involving the nucleus of the VI cranial nerve in an immunocompetent host [30] and multiple involvement of cranial nerves by HHV7 [15] have been reported. CD46, a member of the complement regulatory protein (CPR) family, is a ubiquitously expressed membrane-bound glycoprotein in humans; HHV6 uses CD46 as a receptor mediating membrane fusion with host cells [21]. In addition, this protein prevents complement activation on autologous cells [31]. Elevated CD46 expression on infected T cells contributes to their resistance to complement-dependent cytotoxicity [32, 33]. There are also other immune modulation mechanisms enabling suppression of immune responses in infection: activation of CD4+ and CD8+ T cell proliferation [34-36]; activation of expression of the cytokines that inhibit IL-2 synthesis by activated T cells, and subsequent deactivation of T cells; suppression of the growth and differentiation of marrow progenitors; and reduction of HLA class I expression on dendritic cells [34, 37]. It has been demonstrated that, without viral replication, HHV7, HHV6A and some strains of HHV6B can induce cell-cell fusion between cells expressing human CD46 [38, 39]. Studies [40] have shown that HHV7 and/or HHV6 displayed cytopathic effects, and acute in vitro HHV7 and/or HHV6 infection induced the formation of giant multinucleated syncytia, which eventually underwent necrotic lysis. In addition, apoptosis, another cytopathic effect, was observed in the cells infected with viruses. Infection with HHV6 activates the apoptotic pathway, p53/p21WAF. The apoptotic activation pathway in cells infected with HHV7 is less marked than in those infected with HHV6 [40]. Since nerve cells, astrocytes and oligodendrocytes, also express CD46, they may become infected with HHV6 and HHV7. It had been shown that infected T cells transfer viruses to astrocytes and oligodendrocytes through the interaction between viral glycoproteins expressed on the membrane of the infected cells and CD46 on the glial targets. These data suggest a mechanism that involves cell-cell fusion by which certain viruses could spread the infection from the periphery to the cells in the CNS [41]. In vitro infected cells display characteristic cytopathic effects including the formation of syncytia. The viral particles newly synthesized in the infected cell do not change the type of this cell. Herpesviruses are capable of re-infecting T cells and primary astrocytes [42]. Over 95% of adults are infected with Epstein–Barr virus (EBV) that belongs to the gammaherpesvirus subfamily; most infections occur in young children. The virus affects B lymphocytes [43] and epithelial cells [44]. EBV binds to B-lymphocytes via a viral glycoprotein receptor, gp350/220 which interacts with the complement receptor CD21 (or CR2) [45]. The findings of a recent study [46] suggest that CD35 is another receptor for EBV gp350/220. The precise mechanism of EBV infection of CNS cells has yet to be elucidated. Previously suggested mechanisms include direct viral invasion, secondary autoimmune response, and the mechanism mediated through immune system responses [47-49]. Primary EBV infection causes infectious mononucleosis [50]. Thereafter, the virus generally establishes asymptomatic lifelong persistence in immortalized B lymphocytes. In some cases, most commonly, in the presence of the trigger, EBV may be reactivated and cause other disorders including cancers and CNS disorders [51, 52]. It has been reported that the rate of neurological complications in EBV infections was 7% [53-56]. In addition, cases of isolated CN lesions like unilateral or bilateral facial nerve lesions associated with Epstein-Barr virus infection [16], and CN IV lesion with bilateral acute retinal necrosis following HHV6 and EBV infection of the CNS [17] have been reported. Herpesviruses are the second most common viral infection in humans after the flue. Most herpesvirus infections, however, run a benign or asymptomatic course. Severe herpesvirus infections can occur under immunosuppressive conditions like stress, trauma or other infection. Any individual is at risk of activation of these latent infections. EBV, HHV6, HHV7 have high tropism to cranial nerves (including CN III and VI) which advocates for testing of blood for these viruses in the presence of oculomotor abnormalities of unknown etiology. This is confirmed by the fact that our study patients showed partial or complete resolution of OMA after etiotropic treatment. Conclusion Herpesviruses DNA was detected in venous blood of patients with OMA related to CN III and/or VI lesions of undetermined etiology. HHV7 was most common (45.7% of patients), followed by EBV (14.3%), HHV6 (5.7%), HSV1/2 (1 patient) and CMV (1 patient). Eight patients (22.8%) were found to have two viruses. Of these, 6 had both EBV and HHV7, one had both EBV and HHV6, and one had both HHV6 and HHV7. In addition, 2 patients (5.7%) were found to have three viruses, EBV, HHV6, and HHV7. Normalization of CN functions and resolution of oculomotor abnormalities were accompanied by elimination of viral DNA from blood. For patients with CN III and/or VI dysfunction of unknown etiology, the venous blood should be tested by PCR for herpesviruses after excluding neurosurgical pathology. References 1. Kochina ML, Demin YuA, Kovtun NM, Kaplin IV. [Peculiarities of interferential pictures of eyes at horizontal heterotropy]. Ukrainskiy zhurnal meditsiny, biologii i sportu. 2017 Mar; 2(4):75-81. Russian. 2. Kovtun NM. [Interference Patterns of the Eye Cornea with Different States of Oculomotor Muscle]. Ukrainskiy zhurnal meditsiny, biologii i sportu. 2017; 6(8):81-6. Russian. 3. Jacobson DM, Trobe JD. The emerging role of magnetic resonance angiography in the management of patients with third cranial nerve palsy. Am J Ophthalmol. 1999 Jul;128(1):94-6. 4. Slepowa OS, Svetlova EV, Kovaleva LA, et al. [PCR study of the human herpes virus type 6 and other viruses of the herpes group in eye diseases]. Vopr Virusol. 2015;60(6):45-8. Russian. 5. Zhu L, Zhu H. Ocular herpes: the pathophysiology, management and treatment of herpetic eye diseases. Virol Sin. 2014 Dec;29(6):327-42. doi: 10.1007/s12250-014-3539-2. Epub 2014 Dec15. 6. Sugita S, Shimizu N, Watanabe K, Ogawa M, Maruyama K, Usui N, Mochizuki M. Virological analysis in patients with human herpes virus 6-associated ocular inflammatory disorders. Invest Ophthalmol Vis Sci. 2012 Jul 12;53(8):4692-8. 7. Gonzalez-Gonzalez LA, Molina-Prat N, Doctor P, Tauber J, Sainz de la Maza MT, Foster CS. Clinical features and presentation of infectious scleritis from herpes viruses: a report of 35 cases. Ophthalmology. 2012 Jul;119(7):1460-4. 8. Dyson H, Shimeld C, Hill TJ, Blyth WA, Easty DL. Spread of herpes simplex virus within ocular nerves of the mouse: demonstration of viral antigen in whole mounts of eye tissue. J Gen Virol. 1987 Dec;68 ( Pt 12):2989-95. 9. Shanehsazzadeh M, Rad JS, Pourazar A, Behbahani M. Epidemiology of herpes human virus 6 and 7 infections in salivary gland neoplasms in Isfahan, Iran. Med Arch. 2014 Aug;68(4):276-8. 10. El-Habashi N, Kato Y, El-Nahass E, Fukushi H, Hirata A, Sakai H, Kimura J, Yanai T. An ocular infection model using suckling hamsters inoculated with equineherpesvirus 9 (EHV-9): kinetics of the virus and time-course pathogenesis of EHV-9-induced encephalitis via the eyes. Vet Pathol. 2013 Jan;50(1):56-64. 11. Higaki S, Fukuda M, Shimomura Y. Virological and molecular biological evidence supporting herpes simplex virus type 1 corneal latency. Jpn J Ophthalmol. 2015 Mar;59(2):131-4. 12. Borkar DS, Gonzales JA, Tham VM, Esterberg E, Vinoya AC, Parker JV, Uchida A, Acharya NR. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014 Mar;132(3):326-31. 13. Jester JV, Morishige N, Ben Mohamed L, Brown DJ, Osorio N, Hsiang C, Perng GC, Jones C, Wechsler SL. Confocal Microscopic Analysis of a Rabbit Eye Model of High-Incidence RecurrentHerpes Stromal Keratitis. Cornea. 2016 Jan;35(1):81-8. 14. Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, Jacobson S.. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci USA. 2011 Aug 16; 108(33):13734-9. 15. Velázquez Benito A, Santos Lasaosa S, Viloria Alebesque A, García Arguedas C. Multiple involvement of cranial nerves by an uncommon pathogen: humanherpesvirus type 7. Med Clin (Barc). 2011 Nov 26;137(14):667-8. 16. Grassin M, Rolland A, Leboucq N, Roubertie A, Rivier F, Meyer P. Bilateral facial nerve palsy associated with Epstein-Barr virus infection in a 3-year-old boy. Arch Pediatr. 2017 Jun;24(6):564-567. 17. Papageorgiou E, Ch'ng S, Kulkarni A, Anwar S, Empeslidis T. Fourth cranial nerve palsy and bilateral acute retinal necrosis following human herpesvirus 6 infection of the central nervous system. Ocul Immunol Inflamm. 2014 Jun;22(3):228-32. 18. Tesini BL, Epstein LG, Caserta MT. Clinical impact of primary infection with roseoloviruses. Curr Opin Virol. 2014 Dec;9:91-6. 19. Miyazaki Y, Namba H, Torigoe S, Watanabe M, Yamashita N, Ogawa H, Morishima T, Yamada M. Monitoring of human herpesviruses-6 and -7 DNA in saliva samples during the acute and convalescent phases of exanthem subitum. J Med Virol. 2017 Apr;89(4):696-702. 20. Roush KS, Domiati-Saad RK, Margraf LR, Krisher K, Scheuermann RH, Rogers BB, Dawson DB. Prevalence and cellular reservoir of latent human herpesvirus 6 in tonsillar lymphoid tissue. Am J Clin Pathol. 2001 Nov;116(5):648–654. 21. Krug LT, Pellett PE. Roseolovirus molecular biology: recent advances. Curr Opin Virol. 2014 Dec;9:170-7. 22. Kawamura Y, Nakayama A, Kato T, Miura H, Ishihara N, Ihira M, Takahashi Y, Matsuda K, Yoshikawa T. Pathogenic Role of Human Herpesvirus 6B Infection in Mesial Temporal Lobe Epilepsy. J Infect Dis. 2015 Oct 1;212(7):1014-21. 23. Szewc AM, Taylor S, Cage GD, Jacobsen J, Bulut OP, de Mello Acute Liver Failure in an Adolescent Male Induced by Human Herpesvirus 6 (HHV-6): A Case Report With Literature Review. DE(3). Lab Med. 2018 Mar 21;49(2):165-174. 24. Caruso A, Rotola A, Comar M, Favilli F, Galvan M, Tosetti M, Campello C, Caselli E, Alessandri G, Grassi M, Garrafa E, Cassai E, Di Luca D. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J Med Virol. 2002 Aug ;67(4):528–33. 25. Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K.. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991 Jun; 72(Pt 6):1401-8. 26. Chi J, Gu B, Zhang C, Peng G, Zhou F, Chen Y, Zhang G, Guo Y, Guo D, Qin J, Wang J, Li L, Wang F, Liu G, Xie F, Feng D, Zhou H, Huang X, Lu S, Liu Y, Hu W, Yao K. Human herpesvirus 6 latent infection in patients with glioma. J Infect Dis. 2012 Nov;206(9):1394-8. 27. Luppi M, Barozzi P, Morris C, Maiorana A, Garber R, Bonacorsi G, Donelli A, Marasca R, Tabilio A, Torelli G.. Human herpesvirus 6 latently infects early bone marrow progenitors in vivo. J Virol. 1999 Jan;73(1):754–9. 28. Andre-Garnier E, Milpied N, Boutolleau D, Saiagh S, Billaudel S, Imbert-Marcille BM. Reactivation of human herpesvirus 6 during ex vivo expansion of circulating CD34_haematopoietic stem cells. J Gen Virol. 2004 Nov;85(Pt 11):3333-6. 29. Marseglia L, Manti S, D Angelo G, Stroscio G, Impollonia D, Arena S, Salpietro C, Gitto E. Human herpesviruses-6 and -7 encephalitis in immunocompetent infants: are they really so uncommon? J Biol Regul Homeost Agents. 2016 Oct-Dec;30(4):1131-1136. 30. Riva N, Franconi I, Meschiari M, Franceschini E, Puzzolante C,Cuomo G, Bianchi A(, Cavalleri F, Genovese M, Mussini C. Acute human herpes virus 7 (HHV-7) encephalitis in an immunocompetent adult patient: a case report and review of literature. Infection. 2017 Jun;45(3):385-388. 31. Killick J, Morisse G(1)(2), Sieger D(2), Astier AL(3)(4). Complement as a regulator of adaptive immunity. Semin Immunopathol. 2018 Jan;40(1):37-48. 32. Takemoto M, Yamanishi K, Mori Y. Human herpesvirus 7 infection increases the expression levels of CD46 and CD59 in target cells. J Gen Virol. 2007 May;88(Pt 5):1415-22. 33. Boeckh M, Nichols WG. Immunosuppressive effects of beta-herpesviruses. Herpes. 2003 May;10(1):12-6. PMID: 12749798 34. Nastke MD, Becerra A, Yin L, Dominguez-Amorocho O, Gibson L, Stern LJ, Calvo-Calle JM. Human CD4_ T cell response to human herpesvirus 6. J Virol. 2012 May; 86(9):4776-92. 35. Gerdemann U, Keukens L, Keirnan JM, Katari UL, Nguyen CT, de Pagter AP, Ramos CA, Kennedy-Nasser A, Gottschalk SM, Heslop HE, Brenner MK, Rooney CM, Leen AM.. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood. 2013 Jan ;121(1):207-18. 36. Martin LK, Schub A, Dillinger S, Moosmann A. Specific CD8+ T cells recognize human herpesvirus 6B. Eur J Immunol. 2012 Nov; 42(11):2901-12. 37. Yamanishi K, Mori Y, Pellett PE.. Human herpesviruses 6 and 7. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 2. Lippincott Williams & Wilkins, Philadelphia, PA. 2013; p. 2058–2079. 38. Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 2009 Jul;11(7):1001-6. 39. Tanaka Y, Suenaga T, Matsumoto M, Seya T, Arase H. Herpesvirus 6 glycoproteins B (gB), gH, gL, and gQ are necessary and sufficient for cell-to-cell fusion. J Virol. 2013 Oct;87(19):10900-3. Epub 2013 Jul 24. 40. Secchiero P, Flamand L, Gibellini D, Falcieri E, Robuffo I, Capitani S, Gallo RC, Zauli G. Human Herpesvirus 7 induces CD4(+) T-cell death by two distinct mechanisms:necrotic lysis in productively infected cells and apoptosis in uninfected or nonproductively infected cells. Blood. 1997 Dec 1;90(11):4502-12. PMID: 9373261 41. Cassiani-Ingoni R, Greenstone HL, Donati D, Fogdell-Hahn A, Martinelli E, Refai D, Martin R, Berger EA, Jacobson S. CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell-cell fusion. Glia. 2005 Nov 15;52(3):252-8. 42. He J, McCarthy M, Zhou Y, Chandran B, Wood C. Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol. 1996 Feb;70(2):1296-300. PMID: 8551599 43. Klein G, Klein E, Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun. 2010 May; 396, 67–73. 44. Chesnokova LS, Jiang R, Hutt-Fletcher LM. Viral Entry. Curr Top Microbiol Immunol. 2015;391:221-35. 45. Hannan JP. The Structure-Function Relationships of Complement Receptor Type 2 (CR2; CD21). Curr Protein Pept Sci. 2016 Feb;17(5):463-87. PMID: 26916158 46. Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, Fingeroth JD. Human complement receptor type 1/CD35 is an Epstein-Barr virus receptor. Cell Rep. 2013 Feb; 3,371–85. 47. Gurbuz F, Gurbuz B, Çayir A, Tezer H. Epstein-barr virus encephalitis in infancy. West Indian Med J. 2014 Mar;63(2):206-7. 48. Laurence M, Benito-León J. Epstein-Barr virus and multiple sclerosis: Updating Pender's hypothesis. Mult Scler Relat Disord. 2017 Aug;16:8-14. 49. Ito H, Sayama S, Irie S, Kanazawa N, Saito T, Kowa H, Haga S, Ikeda K. Antineuronal antibodies in acute cerebellar ataxia following Epstein-Barr virus infection. Neurology. 1994 Aug;44(8):1506-7.PMID: 8058157 50. Jenson HB. Epstein-Barr virus. Pediatr Rev. 2011 Sept;32:375–83. 51. Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015 Jan;235(2):323-33. 52. Van Samkar A, Poulsen MNF, Bienfait HP, Van Leeuwen RB. Acute cerebellitis in adults: a case report and review of the literature. BMC Res Notes. 2017 Nov 22;10(1):610. 53. Hussain RS, Hussain NA. Ataxia and Encephalitis in a Young Adult with EBV Mononucleosis: A Case Report. Case Rep Neurol Med. 2013 May;2013:516325. 54. Jha HC, Mehta D, Lu J, El-Naccache D, Shukla SK, Kovacsics C, Kolson D, Robertson ES. Gammaherpesvirus Infection of Human Neuronal Cells. MBio. 2015 Dec 1;6(6):e01844-15. 55. Kano K, Katayama T, Takeguchi S, Asanome A, Takahashi K, Saito T, Sawada J, Saito M, Anei R, Kamada K, Miyokawa N, Nishihara H, Hasebe N. Biopsy-proven case of Epstein-Barr virus (EBV)-associated vasculitis of the central nervous system. Neuropathology. 2017 Jun;37(3):259-264. 56. Mazur-Melewska K, Breńska I, Jończyk-Potoczna K, Kemnitz P, Pieczonka-Ruszkowska I, Mania A, Służewski W, Figlerowicz M. Neurologic Complications Caused by Epstein-Barr Virus in Pediatric Patients. J Child Neurol. 2016 May;31(6):700-8.
|