J.ophthalmol.(Ukraine).2019;6:39-43.
|
http://doi.org/10.31288/oftalmolzh201963943 Received: 17 October 2019; Published on-line: 06 January 2020 Formation of biofilms in traumatic injuries of the ocular adnexa O.V. Petrenko1, Dr. Sc. (Med.), Prof.; M.M. Dranko1, 2, a Postgraduate Student; V.M. Golubnycha3, Cand. Sc. (Biol.); L.V. Grytsai2, Cand.Sc. (Med.) 1 P.L. Shupyk National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 2 Sumy Regional Clinical Hospital, Eye Microsurgery Department; Sumy (Ukraine) 3 Sumy State University, Medical Institute, Public Health Department; Sumy (Ukraine) E-mail: drankoma@ukr.net TO CITE THIS ARTICLE: Petrenko OV, Dranko VV, Golubnycha VM, Grytsai LV. Formation of biofilms in traumatic injuries of the ocular adnexa. J.ophthalmol.(Ukraine).2019;6:39-43. http://doi.org/10.31288/oftalmolzh201963943
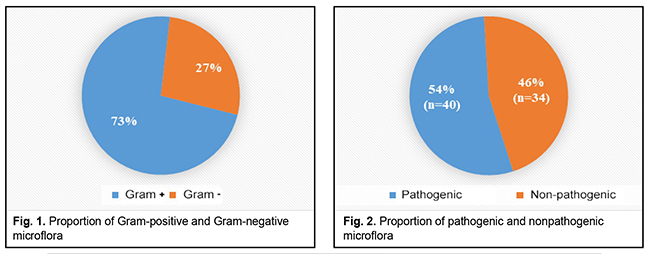
Introduction. Biofilms (microbial communities) can be formed by both pathogenic and non-pathogenic microorganisms. The formation of biofilms causes many problems in the clinical practice as they slow down the healing process and are often characterized by antibiotic resistance. Purpose. To study the ability of microorganisms that were obtained from the wounds of patients with traumatic injuries to the ocular adnexa to form biofilms. Materials and Methods. 60 patients with traumatic ocular adnexal injuries were examined. The bacteriological examination of wound swabs was carried out with species composition and population levels of the microflora defined. The ability of microorganisms to form biofilms was further analyzed. The biofilm studies were performed to determine the amount of biomass generated [O'Toole and Kolter, 1998] by dyeing the biofilms with crystal violet. The method is based on the ability of the crystal violet dye to bind to cells and a matrix of biofilm. The results were interpreted according to the optical density of the dyed solvent. Results. Seventy-five (75) strains of microorganisms were collected and identified from sixty (60) patients. Gram-positive and gram-negative microflora made up 73% (n=54) and 27% (n=20), respectively. The leading positions were taken by Staphylococcus aureus (n=21), Acinetobacter spp. (n=11), Кlebsiellа ozenae (n=8), Micrococcus spp. (n=8), Corynebacterium spp. (n=5), Artrobacter cuminsii (n=4), Staphylococcus epidermidis (n=3). The greatest ability for biofilm formation was manifested in Staphylococcus aureus, Acinetobacter spp. and Klebsiella ozenae, which are the representatives of the opportunistic flora. Staphylococcus aureus was most intensively forming a biofilm in the time interval from Day 1 to Day 3; Acinetobacter spp. was most active during the first day and Klebsiella ozenae – Day 3 to Day 7. Conclusions. Most clinically relevant microorganisms that had been collected from the wounds of patients with traumatic injuries of the ocular adnexa had an ability to form microbial biofilms. This ability was most evident in Staphylococcus aureus, Acinetobacter spp. and Klebsiella ozenae, which are the representatives of the opportunistic flora. Keywords: biofilm, microorganisms, wound infection, ocular adnexa, crystal violet
Introduction In the natural environments, microorganisms are known to exist mostly within microbial communities which are called biofilms. The current concept describes a biofilm as a continuous layer of bacterial cells which stick to each other and a surface and embedded with a biopolymer matrix [1, 4]. Such bacterial organizations can be formed by bacteria of one or several types. They can be comprised of active functioning cells or of “resting” nonculturable bacteria. Biofilms can be formed by nonpathogenic and pathogenic bacteria from the skin and mucosa flora [1, 8]. It has been proved than microorganisms can form biofilms on both biotic and abiotic surfaces [1,8,11]. Microbial biofilms colonize all implants which are used in the medical practice, such as suture material, drainage, contact lenses, intraocular lenses, stents, catheters, artificial valves etc. [1, 5, 11]. This deserves special attention since biofilms can develop on them as early as first days after being implanted. This mode of existence of bacteria, fungi, and microbial associations causes numerous problems in the surgical practice since microbial biofilm formation contributes to inflammation, making it chronic, and significantly slows down the wound healing [5, 7]. In addition, biofilm microorganisms are characterized by high rates of resistance to antibacterial drugs, antiseptics, disinfectants, antibodies, and phagocytes [5, 8]. Experiments have demonstrated that planktonic forms of bacteria and fungi in most cases induce acute inflammation and acute exacerbation of a chronic disease, which can be observed at biofilm rupture and dissemination of the pathogen. While microorganism of biofilms can cause chronic diseases, among which are ocular inflammatory diseases (blepharitis, conjunctivitis, keratitis, scleritis, uveitis), infections of ocular adnexa soft tissues, chronic osteomyelitis etc. [1, 5, 8, 11]. Researchers, including microbiologists, pharmacists, and clinicians, pay much attention to studying biofilms since the ability of pathogenic bacteria to form biofilms creates problems in the clinical practice. Given all of the above and the relevance of this problem, the purpose of our paper was to study the ability of microorganisms that were obtained from the wounds of patients with traumatic ocular adnexal injuries to form biofilms. Materials and methods In 2018-2019, we examined 60 patients with traumatic injuries of the ocular adnexa who applied to the emergency room of Eye Microsurgery Department, Sumy Regional Clinical Hospital. The examinations followed the standards of health care delivery and regulations of the ethics committee. The bacteriological examination of wound swabs was carried out to define the presence of microorganisms in the injured tissues and to study their species composition. The material studied was collected at the patient’s first visit before primary surgical debridement and antibacterial therapy. Species composition and population levels of microflora in the injured tissues were defined following the modification requirements for counting the number of bacteria using the Urquhart and Gould plating method, at the Bacteriological Laboratory of the Sumy State University Medical Institute. Isolates with a bacterial count of 102 CFU/mL were considered diagnostically significant. Species were identified using classical methods for isolation and identification of microorganisms [3]. The microorganisms which were sown more frequently were, afterwards, analyzed for the ability to form biofilms. The amount of biomass generated was determined in the biofilms [O'Toole and Kolter, 1998] [1, 3, 9]. Thus, a mixture of the microorganisms studied was placed into a polystyrene plate and incubated in a nutrition broth for 1, 3, and 7 days at 37?С. After culturing bacteria, the medium with planktonic cells was diluted from the plate and washed thrice in sterile phosphate buffer saline (PBS) at the same amount as the cultivation; PBS was also removed. Afterwards, 4 ml of 0.1% crystal violet were placed into the plate wells. Biofilms were incubated with dye for 10-15 minutes at room temperature. The dye was then removed from the wells which were then washed to remove the non-bonded dye. The plates were turned upside-down onto filter paper and dried. Thereafter, 4 ml of 95% ethanol solution were added to the wells. The solvent was collected and placed in clean flat-bottomed plates and the optical density was measured at a wavelength of 595 nm by a Multiscan FC Microplate Photometer (Thermo Scientific, ESW 1.01.16 version). The results were interpreted according to the optical density of the dyed solvent [1, 3, 9]. The results were processed statistically by the Graph Pad Quik Calcs program with the t-Student test. Results and Discussion 60 patients with traumatic injuries of the ocular adnexa were examined, which revealed and identified 75 strains of microorganisms. A relative ratio of Gram-positive to Gram-negative flora was 73% (n=54) to 27% (n=20), respectively, which is demonstrated in Figure 1.
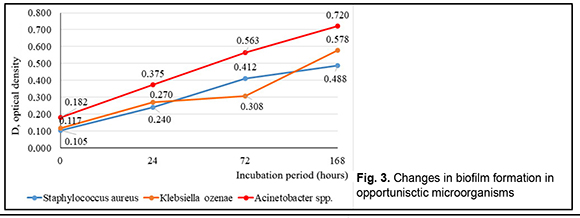
Table 1 shows that the leading positions were taken by Staphylococcus aureus (n=21), Acinetobacter spp.(n=11), Кlebsiellа ozenae (n=8), Micrococcus spp. (n=8), Corynebacterium spp. (n=5), Artrobacter cuminsii (n=4), Staphylococcus epidermidis (n=3). The rest of the strains were revealed infrequently.
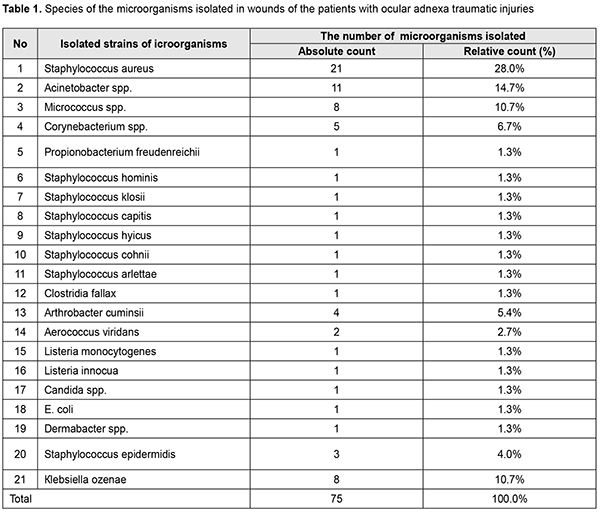
Staphylococcus epidermidis, Micrococcus spp., coryneform bacteria (Corynebacterium spp., Artrobacter cuminsii) are commensal bacteria and cannot cause diseases if the skin is not damaged. Staphylococcus aureus, Acinetobacter spp. and Кlebsiellа ozenae are opportunistic pathogens which can induce various purulent inflammations, from soft tissue wound infection to panophthalmitis, and are often characterized by resistance to antibiotics (Fig. 2) [2, 7].
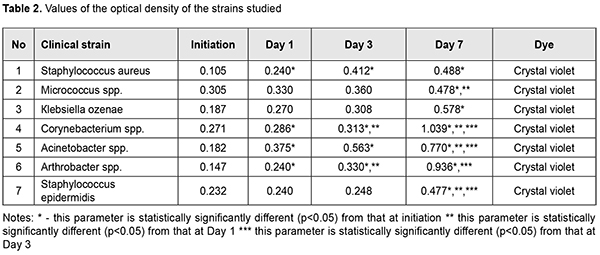
Considering the frequency of microorganism isolation and the ability to form biofilms, we revealed Staphylococcus aureus, Acinetobacter spp., Кlebsiellа ozenae, Micrococcus spp., Corynebacterium spp., Arthrobacter cuminsii, and Staphylococcus epidermidis. Based on the data in Table 2, it can be concluded that most microorganisms form biofilms in vitro. Most of all, this ability is shown in Staphylococcus aureus, Acinetobacter spp. and Кlebsiella ozenae, which are opportunistic pathogens. The rest of the strains are characterized by a weaker ability to form biofilms. The difference was considered to be statistically significant with p<0.05.Figure 3 demonstrates the changes in forming biofilms by opportunistic pathogens.
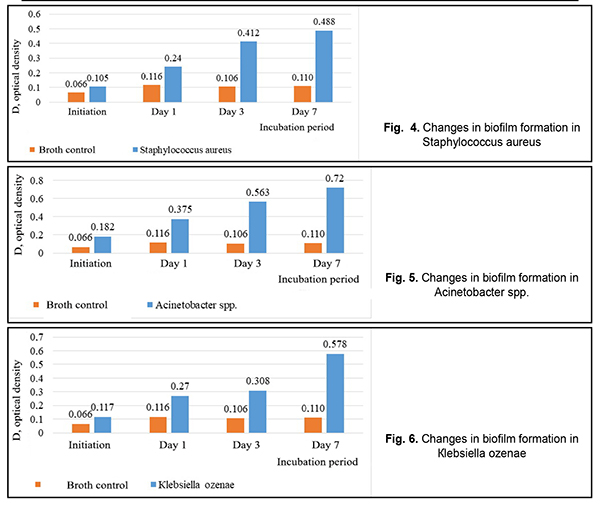
In Figure 4, it can be seen that Staphylococcus aureus was forming a biofilm at the most intensive rate within the time period from Day 1 to Day 3. Figure 5 demonstrates that the most intensive rate of biofilm formation for Acinetobacter spp. was on Day 1. Figure 6 shows that Кlebsiella ozenae was forming a biofilm most actively from Day 3 to Day 7. Experiments have demonstrated that the ability of microorganisms to form a biofilm can be realized not only in vitro but also directly in wounds [5, 6, 7, 8]. It is also known that the planktonic forms of bacteria and fungi in most cases induce acute inflammation and acute exacerbation of a chronic disease, which can be observed at biofilm rupture and dissemination of the pathogen, while microorganism of biofilms can cause chronic diseases [4, 6, 7]. Since bacteria in biofilms are resistant to both antimicrobial drugs and nonspecific defences, one of the directions in treating wound infection must be the inhibition of microorganisms’ ability to form a biofilm and lysis of existing biofilms [4, 7, 10]. Conclusions Firstly, Staphylococcus aureus (n=21), Acinetobacter spp. (n=11), Кlebsiellа ozenae (n=8), Micrococcusspp. (n=8), Corynebacterium spp. (n=5), Arthrobacter cuminsii (n=4), Staphylococcus epidermidis (n=3) were sown more frequently in the patients with traumatic injuries of the ocular adnexa. Other strains were sown rarely (p<0.05). Secondly, most clinically significant microorganisms obtained from the wounds of the patients with ocular adnexal injuries had an ability to form biofilms. Thirdly, most of all this ability was manifested in Staphylococcus aureus, Acinetobacter spp. and Кlebsiella ozenae, which are representatives of the opportunistic flora. Finally, Staphylococcus aureus was most intensively forming a biofilm in the time interval from Day 1 to Day 3; Acinetobacter spp. was most active during the first day and Klebsiella ozenae – from Day 3 to Day 7. References: 1.Mardanova AM, Kabanov DA, Rudakova NL, Sharypova MR. [Biofilms: basic research methods: study guide]. Kazan: К(P)FU; 2016. 42 p. In Russian. 2.Yuzhakov AM, Hundorova RA, Neroev VV, Stepanov AV. [Intraocular Wound Infection: Guideline for Physicians]. Moscow: Ltd. Meditsinskoe informatsionnoe agenstvo; 2007. 240p. In Russian. 3.Liuta VA, Kononov OV. [Microbiology with microbiological research technique, virology and immunology: A Textbook (For higher education institutions I–III levels of accreditation). 2nd ed.] Kyiv: Medytsyna; 2018. 576 p. In Ukrainian. 4.Nedashkivska VV, Dronova ML, Vrynchanu NO. [Biofilms and their role in infectious diseases]. Ukrayinskiy naukovo-medichniy molodizhniy Zh. 2016; 98(4):11-19. In Ukrainian. Available from: https://msgl-journal.com/index.php/journal/article/download/85/84 5.Okulych VK, Kabanova AA, Plotnikov FV. [Microbal biofilms in clinical microbiology and antibiotic therapy]. Vitiebsk: VGMU; 2017. 300 p. In Russian. 6.Osypova EV, Shypytsyna YV, Naumenko ZS. [A comparative quantitative evaluation of the potential of biofilm formation by different bacterial clinical strains on polystirole and glass surface]. Mezhdunarodnyi zhurnal prikladnyh i fundamentalnyh issledovaniy. 2014;8-1:55-58. In Russian. Available from: https://applied-research.ru/ru/article/view?id=5638 7.Petrenko OM, Bezrodnyi BH, Bondarchuk OL. [Formation of biofilms by soft tissue phlegmon]. Khirurhiya Ukraini. 2016;1:85-89. In Ukrainian. Available from: http://nbuv.gov.ua/UJRN/KhU_2016_1_16 8.Sidashenko O., Voronkova O., Sirokvasha O., Vinnikov A. [Methods to study the dynamics biofilm-formation of opportunistic bacteria]. Visnyk Lvivskoho Universitetu. Series Biology. 2014;65:20-33. In Ukrainian. Available from: http://prima.lnu.edu.ua/faculty/biologh/wis/65/0/2/2.pdf 9.Symonova YR, Holovyn SN, Verkyna LM, Berezniak EA, Tytova SV. [Methods of culturing and studying bacterial biofilms]. Izvestiya Vuzov. Severo-Kavkazskii Region. 2017;1:73-79. In Russian. Available from: https://lib.rucont.ru/efd/597919/info 10.Chebotar YV. [Biofilms of Staphylococcus aureus: structural and functional characteristics and the relationship with neutrophils: a dissertation for the degree of Doctor of Medicine]. N. Novgorod. 2014: 43. In Russian. 11.Percival SL, McCarty SM, Lipsky B. [Biofilms and Wounds: An Overview of the Evidence]. Advances in Wound Care. 2015;4(7):373-381. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|