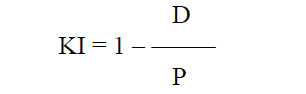J.ophthalmol.(Ukraine).2020;2:36-44.
|
http://doi.org/10.31288/oftalmolzh202023644 Received: 21 February 2020; Published on-line: 30 April 2020 Pupil response to accommodation and convergence in healthy children and adolescents of various age groups and autonomic nervous system tone Shakir Dukhayer, Ophthalmologist; N.M. Bushuieva, Dr Sc (Med); S.B. Slobodianyk, Cand Sc (Med) SI "Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: bushuyevan@gmail.com TO CITE THIS ARTICLE: Shakir Dukhayer, Bushuieva NM, Slobodianyk SB. Pupil response to accommodation and convergence in healthy children and adolescents of various age groups and autonomic nervous system tone. J.ophthalmol.(Ukraine).2020;2:36-44. http://doi.org/10.31288/oftalmolzh202023644
Background: As there is close association between pupil response and accommodation during gaze fixation on an object at any distance, it is important to assess accommodative pupil response both in healthy children and in those with accommodative disorders. It is also important to assess relationships of characteristics of pupil response to accommodation and convergence with age and autonomic nervous system (ANS) tone. It is hypothesized that pupillography parameters can be regarded as objective criteria for assessing pupil response to accommodation and convergence, and serve as an important consideration in choosing the treatment method in patients with accommodative disorders. Purpose: To assess pupil response to accommodation and convergence in healthy children and adolescents of various age groups and autonomic nervous system (ANS) tones. Material and Methods: Pupil response to accommodation and convergence was studied in 269 systemically and ophthalmologically healthy children and adolescents (538 eyes) aged 6 to 18 years. Subjects were divided into three groups based on their age: group 1 (6-9 years; 77 subjects), group 2 (10-14 years; 96 subjects), and group 3 (15-18 years; 96 subjects). ANS tone was assessed using the Kerdo index. Pupillography studies were performed using an OK-2 pupillographer (Odesa, Ukraine). Pupil area, amplitude of change in pupil area, and duration of phases of change in pupil area were determined. Results: We determined normative values for pupillographic characteristics for 6-9, 10-14 and 15-18 years age groups with various ANS tones (increased parasympathetic, normal or increased sympathetic tone). In addition, we determined whether these characteristics depend on the child’s age and/or balance of autonomic innervation. There was pupillographic evidence that those with increased sympathetic tone had significantly greater pupil area and amplitude of changes in pupil area, and significantly shorter timing of changes in pupil size than those with normal ANS tone or increased parasympathetic tone. The fact that younger children (i.e., those aged 6-9 years) had slower pupil response to accommodation and lower amplitude of change in pupil area compared to older children and adolescents (i.e., those aged 10-18 years) may indicate a low level of structural and functional maturity of the accommodative and convergence system at this age. Conclusion: The patterns of relationships revealed in the study provide grounds for regarding pupillography data as an objective criterion for assessing the pupil response to accommodation and convergence and the balance of ANS innervation in children and adolescents of various age groups. Keywords: pupillography, accommodation, pupil response to accommodation and convergence, children and adolescents
Introduction Accommodation is the ability of the eye to adjust its refractive power to keep an object in focus at various distances [1]. The mechanism of accommodation is implemented through a number of structural-and-functional components (the crystalline lens, ciliary muscle, and choroid) which are controlled by the sympathetic and parasympathetic compartments of the autonomic nervous system (ANS) in the process of accommodation. Accommodation is closely associated with pupil response; both of them are components of the unconditioned reflex which is implemented through the activity of the accommodative and convergence system. The system provides simultaneous achievement of best-corrected visual acuity, binocular vision, and correct position of the eyes focused on objects at any distance. Provision of increased focus for near vision is the main function of pupillary response which is a component of accommodative and convergence reflex, and is expressed through pupillary constriction that occurs when a person changes his gaze from far to near [1, 2, 3]. Knowledge of the state and response of the pupillary system is very important for determining a variety of the disorders of the visual system, ANS and central nervous system (CNS). That is why pupillometry as an objective research technique is used to (1) diagnose conditions and disorders of the CNS, (2) identify functional impairments in persons with eye pathology, including glaucoma, diabetic retinopathy, pigmented retinitis, retinitis pigmentosa, strabismus, and amblyopia, and (3) assess individual’s capacity for visual work, etc. [4-14]. As there is close association between pupil response and accommodation during gaze fixation on an object at any distance, it is important to assess accommodative pupil response both in healthy children and in those with accommodative disorders. The prevalence of the latter was 3.68 per 1000 children aged under 6 years, 35.57 per 1000 children aged 7–14 years, and 84.86 per 1000 children aged 15–17 years in Ukraine in 2018 [15]. It is also important to assess relationships of characteristics of pupil response to accommodation and convergence with age and ANS tone. It is hypothesized that pupillography parameters can be regarded as objective criteria for assessing pupil response to accommodation and convergence, and serve as an important consideration in choosing the treatment method in patients with accommodative disorders. The purpose of the first stage of this study was to determine normative pupillography values. The purpose of the study was to assess pupil response to accommodation and convergence in healthy children and adolescents of various age groups and autonomic nervous system tones. Material and Methods Pupil response to accommodation was studied in 269 healthy children and adolescents (538 eyes) aged 6 to 18 years. All study subjects were systemically and ocularly healthy. Eye examination showed that children and adolescents had central eye fixation, binocular vision, mean visual acuity for near and distance of 1.02±0.02, and no pathological changes in the anterior eye, optic media or fundus. Refractive error was normal for age, and mean corneal refractive power was 42.83 ± 0.10 D. The mean axial length as measured by ultrasound biometry was 23.4±0.25 mm. Subjects were divided into three groups based on their age: group 1 (6-9 years; 77 subjects), group 2 (10-14 years; 96 subjects), and group 3 (15-18 years; 96 subjects). Group age ranges were selected based on previously reported findings on changes in the balance of autonomic pupil innervation in ontogenesis. Thus, Velkhover and colleagues [4] found that, in children, mydriasis begins progressing at the age of 2 months of their life, and this progression continues for 10 years at average, indicating that the sympathetic ANS develops at a higher rate than the parasympathetic ANS. Approximately at the age of 10-14 years, the sympathetic ANS activity approaches its maximum, and an approximate balance is achieved in reciprocal interaction between cholinergic (parasympathetic) and adrenergic (sympathetic) components of ANS innervation of the sphincter, and, probably, accommodative muscle [4, 5]. Subsequently, the activity of the parasympathetic component increases, approaching its maximum by 20-24 years of age. ANS tone was assessed using the ANS dysfunction questionnaire designed by Vein and the Kerdo index (KI) [16]. The subjects completed the questionnaire by answering either “Yes” of “No” to the questions regarding the function of their ANS, and a total score was computed by summing all positive answers. The total score for healthy individuals was 15 or less. The Kerdo index (KI) comprising cardiovascular characteristics, blood pressure and cardiac frequency (or heart rate, beats/min), was used to determine whether sympathetic or parasympathetic ANS tone was apparent [16]. This study was performed with the patient sitting; heart rate (beats/min) was measured at the radial artery, and blood pressure measurements (mmHg) were performed in a standard manner. Kerdo index (KI) was calculated with the following formula:
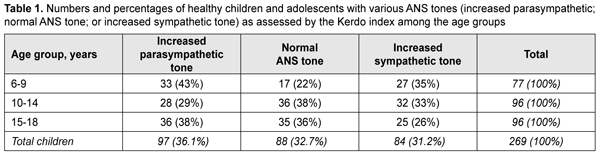
where D is diastolic blood pressure (mmHg) and P is heart rate (beats/min). The Kerdo index value was used to make a conclusion on the balance of sympathetic and parasympathetic effects on the cardiovascular system. KI equals zero in normal autonomic nervous system tone, is negative in individuals with an increased parasympathetic tone, and positive in those with an increased sympathetic tone of the autonomic nervous system. Pupillography studies were performed using an OK-2 pupillographer (Ukraine) developed by the Filatov institute in cooperation with Om-Tekhnologiia (Odesa, Ukraine) [17]. Pupillometry studies of pupil response to accommodation and convergence were conducted using our methodology reported previously [17, 18]. Data were obtained in a dark clinic room throughout 9:00 AM – 11:00 AM. In brief, each subject was adapted to a background illumination (10 lux; 15 minutes) and instructed regarding the pupillometry procedure prior to the start of the study session. Thereafter, he had a headpiece with IR video cameras put on, and the video recording was switched on. During video recording of pupil response to accommodation, a background illumination was 10 lux. The subject fixed his eyes on a test object located at a distance of 100 cm (relaxed accommodation and convergence), and shifted his gaze to a test object located at a distance of 10 cm (strained accommodation and convergence). Video recording data were processed by software to produce a plot of changes in the pupil area for both eyes for the study protocol. In addition, direct and consensual pupil responses to light stimuli (50 lux light intensity; 12 s duration) at a background illumination of 10 lux were studied to compare pupillography indices for various types of pupil responses. The following pupil response characteristics were subjected to analysis: maximum pupil area (Smax, under relaxed accommodation), minimum pupil area (Smin, pupil constriction under strained accommodation or due to light effect), amplitude of change in pupil area (A), and duration of phases of change in pupil area (Phase II, pupil constriction latency; Phase III, active pupil constriction; Phase V, pupil re-dilation latency; and Phase VI, fast pupil re-dilation). Statistical analyses were conducted using Statistica 8 (StatSoft, Tulsa, OK, USA) and Microsoft Excel 2007 software. Mean values, standard deviations (SD), and their 95% confidence intervals and significance values (p) were calculated. A two-way analysis of variance and Fischer’s F-test were used to determine the effect of the age and state of the ANS on pupillography indices. The level of significance p < 0.05 was assumed. The non-parametric Spearmen test was used to calculate correlation coefficients; these were considered significant at p < 0.05 [19, 20]. Results At baseline, ANS balance was assessed using the Kerdo index. In children of 6-9 years, increased parasympathetic tone was the most common (43%), followed by increased sympathetic tone (35%) and normal ANS tone (22%). In children of 10-14 years, normal ANS tone was the most common (38%) followed by increased sympathetic tone (33%) and increased parasympathetic tone (38%). In adolescents of 15-18 years, increased parasympathetic tone was the most common (38%), followed by normal ANS tone (36%) and increased sympathetic tone (26%) (Table 1).
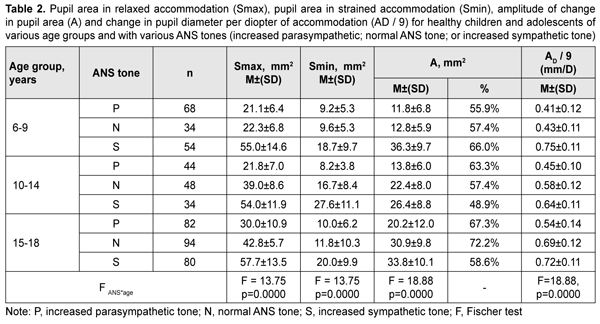
Table 2 presents values of pupil areas in accommodative pupil response for children of various ages and ANS tone. With a test object located at a distance of 100 cm, maximum pupil area (Smax) under relaxed accommodation significantly depended on the child’s age and ANS tone (F=13.75, p=0.0000). The greatest Smax values were found for children with increased sympathetic tone, and the lowest, for those with increased parasympathetic tone (p < 0.001). It should be noted with regard to distribution of Smax values that Smax did not depend on the age, with the mean value being 55.6 ± 13.3 mm2. In addition, among children with normal ANS tone, the Smax gradually increased with the age, from 22.3 ± 6.8 mm2 for children aged 6-9 years, to 39.0 ± 8.6 mm2 and 42.8 ± 5.7 mm2 for those aged 10-14 years and older 14 years, respectively. Moreover, children with increased parasympathetic tone exhibited almost equal levels of Smax, with the mean value being 25.8 ± 8.8 mm2.
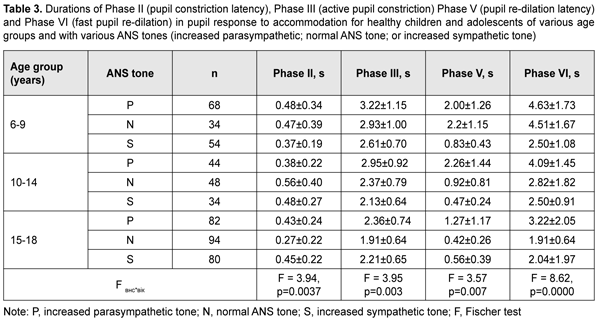
With a test object located at a distance of 10 cm (i.e., under conditions of strained accommodation), minimum pupil area (Smin) also significantly depended on the child’s age and ANS tone (F=13.75, p=0.0000). The greatest Smin values were found for children with increased sympathetic tone, and the lowest, for those with increased parasympathetic tone (p < 0.001) (Table 2). It should be noted that Smin did not depend on the age among children with increased parasympathetic tone, with the mean value being 9.4 ± 1.1 mm2. Among children with normal ANS tone, those of 10-14 years exhibited the greatest mean Smin (16.7 ± 8.4 mm2), followed by adolescents of 15-18 years (11.8 ± 10.3 mm2) and children of 6-9 years (9.6 ± 5.3 mm2). Children with increased sympathetic tone exhibited the same pattern, although with greater Smin values: those aged 10-14 years exhibited the greatest mean Smin (27.6 ± 11.1 mm2), followed by adolescents aged 15-18 years (20.0 ± 9.9 mm2) and children aged 6-9 years (18.7 ± 9.7 mm2). The greatest values of amplitude of changes in pupil area (A) under strained accommodation conditions were found for children with increased sympathetic tone, and the lowest, for those with increased parasympathetic tone (F=18.88, p=0.0000) (Table 2). In general, the mean A value gradually increased with the age, especially for those with increased parasympathetic tone (from 11.8 ± 6.8 mm2 for children aged 6-9 years, to 13.8 ± 6.0 mm2 and 20.2±12.0 mm2 for those aged 10-14 years and older 14 years, respectively) and those with normal ANS tone (from 12.8 ± 5.9 mm2 to 22.4 ± 8.0 mm2 and 30.9 ± 9.8 mm2, respectively). There was, however, no significant difference in the percentage ratio of amplitude of change in pupil area (A) to baseline pupil area (Smax). In addition, the ratio varied from 48.9% to 72.2% depending on child’s age and ANS tone, with mean values of 59.8%, 62.3% and 57.8% for the age groups of 6-9 years, 10-14 years and 15-18 years, respectively. The change in pupil diameter per diopter of accommodation was calculated for accommodative response based on the change in amplitude of pupil area. The eye is known to require 1.0 D and 10.0 D of accommodation during gaze fixation on an object located at 100 cm and 10 cm, respectively [2]. That is, when a person changes his gaze from an object at 100 cm to that at 10 cm, the accommodative and convergence system expends 9.0 D. Therefore, the change in pupil area per diopter of accommodation is A/9 (mm2/D). The change in pupil area (and diameter) per diopter of accommodation significantly depended on the child’s age and ANS tone (Table 2), and its highest value, 3.25 ± 0.46 mm2/D (0.70 ± 0.11 mm/D) was found for children with increased sympathetic tone, followed by those with normal ANS tone, 2.44 ± 0.87 mm2/D (0.57 ± 0.12 mm/D) and increased parasympathetic tone 1.46 ± 0.91 mm2/D (0.46 ± 0.12 mm/D) F=18.88, p=0.0000. Table 3 presents timing data with regard to changes in pupil area during pupil response to accommodation and convergence.
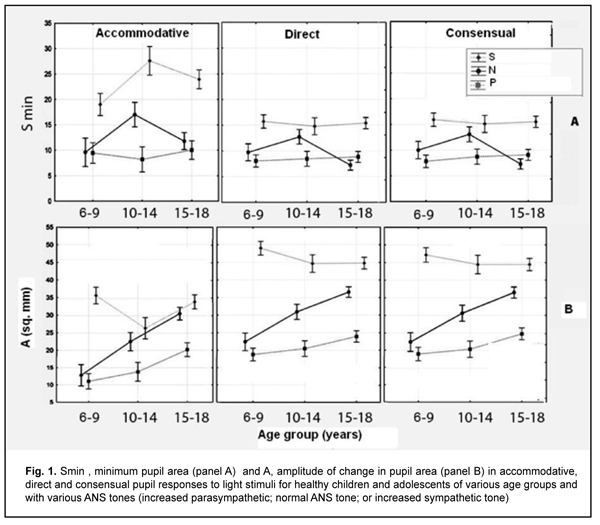
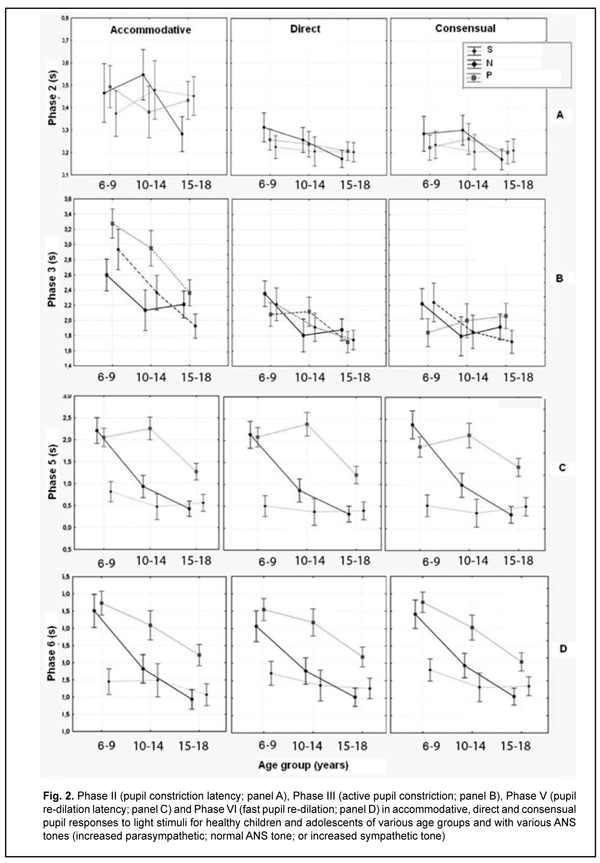
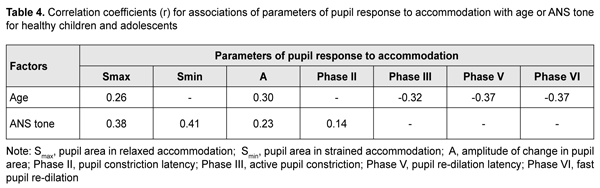
Duration of Phase II (pupil constriction latency) depended on the child’s age and ANS tone (F=3.94, p=0.0037), although its values were unevenly distributed across the study sample, with mean value varying from 0.27 ± 0.22 s to 0.56 ± 0.40 s for the groups (Table 3). Phase III (active pupil constriction) was the longest for children with increased parasympathetic tone (2.84 ± 0.93 s), followed by those with normal ANS tone (2.40 ± 0.80 s) and increased sympathetic tone (2.32 ± 0.65 s) (F=3.57, p=0.007). In addition, children aged 6-9 years exhibited the longest Phase III values (2.92 ± 0.95 s), followed by children aged 10-14 years (2.48 ± 0.78 s) and adolescents aged 15-18 year (2.16 ± 0.69 s) (p = 0.0001). Durations of Phase V (pupil re-dilation latency) and Phase VI (fast pupil re-dilation) also depended on the child’s age and ANS tone (F=3.57, p=0.007 and F=8.62, p=0.0000, respectively), and these phases were longer for children with increased parasympathetic tone than for those with normal ANS tone and increased sympathetic tone (Table 3). It is noteworthy that a significant decrease in Phase 5 duration with age was observed in the presence of normal ANS tone or increased parasympathetic tone, but Phase 5 duration in the presence of increased sympathetic tone was approximately equally low among the three age groups. Compared to children with normal ANS tone or increased parasympathetic tone, those with increased sympathetic tone exhibited shorter Phase 5 and Phase 6 durations, and less variability in these durations with child’s age. Overall, children with increased sympathetic tone exhibited the shortest Phase 2, Phase 3, Phase 5 and Phase 6 durations, and the greatest amplitude of change in pupil area (A), whereas children with increased parasympathetic tone exhibited the longest Phase 2, Phase 3, Phase 5 and Phase 6 durations, and the least amplitude of change in pupil area (A). There were positive correlations between the child’s age and both Smax (r=0.26) and А (r=0.30), (p < 0.05), and negative correlations between child’s age and durations of Phase 3 (r= -0.32), Phase 5 (r= -0.37), and Phase 6 (r = -0.37) (p<0.05). In addition, there were positive correlations between the ANS tone and Smax (r=0.38), Smin (r=0.41), A (r=0.23), and duration of Phase 2 (r=0.14, p<0.05). This indicates that, compared to the age, the ANS tone exerts greater effect on pupil dimensions, and less effect on pupil constriction and re-dilation characteristics in healthy children and adolescents. Therefore, for healthy children aged 5-18 years, there was pupillographic evidence that those with increased sympathetic tone had significantly greater pupil area and amplitude of changes in pupil area, and significantly shorter timing of changes in pupil size than those with normal ANS tone or increased parasympathetic tone. In addition, children with increased parasympathetic tone exhibited the lowest rate and amplitude of change in pupil area. The fact that younger children (i.e., aged 6-9 years) had slower pupil response and lower amplitude of change in pupil area compared to older children and adolescents (i.e., aged 10-18 years) may indicate a low level of structural and functional maturity of the accommodative and convergence system at this age. We also conducted a comparative analysis of accommodative pupil response, direct pupil response and consensual pupil response characteristics for healthy children and adolescents (Figs. 1 and 2). There was no difference in pupillogram characteristics between direct pupil response and consensual pupil response. There was a difference in minimum pupil area (Smin), amplitude of change in pupil area (A) (Fig. 1 A,B), and, especially, durations of Phase II (pupil constriction latency) and Phase III (active pupil constriction) between accommodative pupil response and direct or consensual pupil response (p < 0.05) (Fig. 2 А, B). All the three types of pupil response were similar to each other with regard to Phase V (pupil re-dilation latency) and Phase VI (fast pupil re-dilation) (Fig. 2 C, D).
Discussion This study for the first time reports the following normative indices of pupil response to accommodation and convergence which characterize pupil area and change in pupil area in strained accommodation for healthy children and adolescents of various age groups (6-9, 10-14 and 15-18 years) and ANS tones: maximum pupil area (Smax), minimum pupil area (Smin), amplitude of change in pupil area (A), and duration of phases of change in pupil size (Phase II, pupil constriction latency; Phase III, active pupil constriction; Phase V, pupil re-dilation latency; and Phase VI, fast pupil re-dilation). Previously, most studies have focused on assessment of static pupillography indices such as pupil diameter and pupil area. There is a limited number of studies focusing on dynamic pupillography parameters [4, 5, 21-23], however, without consideration of the balance of autonomic innervation. There is, however, an opinion that some dynamic pupillography parameters (e.g., pupil constriction velocity) depend on the balance between sympathetic and parasympathetic tones. When there is such a balance, an increased sympathetic tone decreases the pupil constriction velocity, whereas an increased parasympathetic tone decreases the velocity. Velkhover and colleagues [4, 5] measured pupil area in healthy individuals aged from birth to 80 years, and found that mean pupil area in the 6-9, 10-14, and 15-19 years age groups was 19.2 ± 0.29 mm2, 19.7 ± 0.29 mm2, and 16.46 ± 0.27 mm2, respectively. They explained such a pattern of pupil area value distribution by the features of ANS development in ontogenesis: before 10-14 years of age, the sympathetic component develops significantly faster than the parasympathetic component. At 10-14 years of age, sympathetic component activity achieves its maximum, with some balance between the two components. Thereafter and until 20-24 years of age, the activity of the parasympathetic component increases. Those authors, however, did not assess the relationship of pupil area with the balance of autonomic innervation. Others [24-27] not only reported that age is a factor significantly influencing pupil characteristics, but also demonstrated that pupil size tends to decrease with age. There is a limited number of studies [13, 28, 29] focusing on age-related changes in pupil response, including velocity of constriction and subsequent dilation of the pupil. Thus, Tekin and colleagues [29] demonstrated that resting diameter, velocity of pupil contraction, and velocity of pupil dilation values were inversely and moderately correlated with age (p < 0.001, r = -0.63; p < 0.001, r = -0.47; and p < 0.001, r = -0.34, respectively), whereas latency of pupil contraction was positively and moderately correlated with age (p = 0.002, r = 0.29) in healthy individuals aged 6 to 70 years. Volkova [30] has noted the effect of autonomic nervous system tone on pupil diameter and found that emmetropes with an increased sympathetic tone exhibited larger pupil diameter (5.17 mm) than those with normal ANS tone (4.33 mm) or those with an increased sympathetic tone (4.08 mm), which is confirmed by our findings. That author, however, did not report on pupil size for different age groups. In the current study, the absolute value of amplitude of change in pupil area was substantially less in accommodative and convergence pupil response than in direct or consensual pupil response. The mean normalized value of amplitude of change in pupil area clearly tended to be less in accommodative pupil response (48.9% to 66% in various age groups) than in pupil response to light stimulus (67.1%-83.6%), which indicated that (1) a pupil excursion in the former response is less than in the latter response, and (2) these two types of pupil response are definitely formed by the mechanisms differing from each other. We found no significant relationship between the normalized value of amplitude of change in pupil area and the age or balance of autonomic innervation. Our findings are similar to those reported by Tekin et al [29], who also found no significant effect of age on the amplitude of pupil contraction presented in percentage of the baseline pupil diameter. Overall, in the current study, there was a similarity between direct and consensual pupil response in terms of all examined parameters, but the was a significant difference between accommodative response and direct or consensual pupil response in terms of the amplitude of change in pupil area (A) and duration of Phase II (pupil constriction latency) and Phase III (active pupil constriction); these phases were substantially longer in accommodative response than in direct or consensual pupil response. The revealed differences may indicate that pupil contraction mechanisms in accommodation and light stimulation of the pupil differ from each other. Our findings somewhat correspond to those reported by Math?t S. [23], with Phase II (pupil constriction latency) and Phase III (active pupil constriction) being substantially longer in accommodative pupil response (0.6 s and 0.6 s – 2 s, respectively) than in pupil response to light stimuli (0.2 s and 0.2 s – 1.5 s, respectively). That author, however, did not report on pupil constriction latency values for various age groups, and noted that the pupil near response (i.e., accommodative response) “is certainly the least studied, and perhaps the least understood of all pupil responses” [23]. Provision of increased focus for near vision is the main function of the accommodative pupil response which is a component of the accommodation and convergence reflex. It has been reported that the neural basis of the pupil response to accommodation is somewhat different from that of the pupil response to light stimuli, which may explain a difference in values of pupillography indices between these two responses. In particular, unlike the pupil light response, the pupil near response does not appear to be driven directly by a subcortical pathway, but rather by cortical projections to the Edinger-Westphal nucleus [31]. Which cortical areas are involved in the pupil near response is not entirely clear; however, there are projections from the frontal eye fields and parietal cortex to the Edinger-Westphal nucleus that are involved in vergence movements [32]. Because of the strong association between vergence and the pupil response to accommodation, and the central role of the Edinger-Westphal nucleus in pupil constriction, it is possible that these projections also play a role in the accommodative pupil reflex [23]. We also calculated the change in absolute value of pupil area (A) per diopter of accommodation occurring when a person changes his gaze from an object at 100 cm to that at 10 cm, A/9 (mm2/D), and demonstrated that it significantly depended on the child’s age and ANS tone. Children with increased sympathetic tone had the largest mean change in absolute value of pupil diameter per diopter of accommodation (0.70 ± 0.11), followed by those with normal ANS tone (0.57 ± 0.12) and with increased parasympathetic tone (0.46 ± 0.12). The present findings are close to those reported by Kasthurirangan and colleagues [21] for a change in pupil diameter per diopter of accommodation in human subjects aged 14–45 years. They found that the mean change in pupil diameter per diopter of accommodation was 0.58 mm/D (range, 0.20 to 0.76 mm/D). Those authors, however, did not report on whether the ANS tone had an effect on the amplitude of pupil response. Conclusion This study focused on healthy children and adolescents aged 6-18 years. We determined normative values for pupillographic characteristics for 6-9, 10-14 and 15-18 years age groups with various ANS tones (increased parasympathetic, normal or increased sympathetic tone). In addition, we determined whether these characteristics depend on the child’s age and/or balance of autonomic innervation. First, there was pupillographic evidence that those with increased sympathetic tone had significantly greater pupil area and amplitude of changes in pupil area, and significantly shorter timing of changes in pupil size than those with normal ANS tone or increased parasympathetic tone. Second, the fact that younger children (i.e., aged 6-9 years) had slower pupil response to accommodation and lower amplitude of change in pupil area compared to older children and adolescents (i.e., aged 10-18 years) may indicate a low level of structural and functional maturity of the accommodative and convergence system at this age. Finally, the patterns of relationships revealed in the study provide grounds for regarding pupillography data as an objective criterion for assessing the pupil response to accommodation and convergence and the balance of ANS innervation in children and adolescents of various age groups. References 1.Katargina LA, editor. [Accommodation: A guide for physicians]. Moscow: Aprel; 2012. Russian. 2.Von Noorden GK, Campos EC, editors. Binocular Vision and Ocular Motility. Theory and management of strabismus. 6th ed. St. Louis; Sydney: Mosby; 2008. 3.Miller NR, Newman NJ, Biousse V, Kerrison JB. Walsh & Hoyt’s Clinical Neuro-ophthalmology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. 4.Velkhover ES, Ananin VF. [Introduction to iridology. Pupillodiagnosis]. Moscow: UDN; 1991. Russian. 5.Velkhover ES, Shulpina NB, Aliieva ZA, Romashov FN. [Iridodiagnosis]. Moscow: Meditsina; 1988. Russian. 6.Boychuk IM, Bushuyeva NN, Romanenko DV. [Pupil reaction with concomitant horizontal and vertical deviation]. Int J Neuro-Ophthalmology. 2010;34:236. 7.Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008 Jul;45(4):602-7. 8.Bushuyeva NN, Chramenko NI, Boichuk IM, Duhair SMH. Computer pupillography in patients with disturbances of cerebral vessels. Neuro-Ophthalmology Society (EUNOS) 11th Meeting Oxford (Oxford, April 10-13, 2013). Neuroophthalmology. 2013; 37(sup1): 1–109. 9.Bushuyeva NN, Boychuk IM, Duhairmh Shaker MH. Particuliarities of pupillogrames in children and adults with hypermetropic amblyopia. Acta Ophthalmologica Scandinavica. 2006;84:52. 10.Girkin CA. Evaluation of the pupillary light response as an objective measure of visual function. Ophthalmol Clin North Am. 2003 Jun;16(2):143-53. 11.Kelbsch C, Strasser T, Chen Y, et al. Standards in Pupillography . Front Neurol. 2019 Feb 22;10:129. 12.Levatin P. Pupillary escape in diseases of the retina or optic nerve. Arch Ophthalmol. 1959;62:768-9. 13.Loewenfeld IS. The Pupil: Anatomy, Physiology, and Clinical Applications. Oxford: Butterworth-Heinemann; 2 edition, 1999. 14.Wilhelm H, Wilhelm B. Clinical Applications of Pupillography. J Neuro-Ophthalmol. 2003 Mar;23(1):42-9. 15.Moiseienko RO, Golubchikov MV, Mikhalchuk VM, et al. [Ophthalmological care in Ukraine in 2014-2017: Analytical and statistical reference book]. Kropivnytskyi: Polium;2018. Ukrainian. 16.Vein AM, editor. [Autonomic nervous system abnormalities: Clinical signs, diagnosis and treatment]. Moscow: Meditsinskoe informatsionnoe agenstvo; 2003. Russian. 17.Bushuieva NM, Boychuk IM, Dukhayer Shakir, Khramenko NI, Ponomarchuk VS. [Method of computer pupillography]. Ukrainskyi medychnyi almanakh. 2006;9(2):24-7. Ukrainian. 18.Bushuieva NM, Boychuk IM, Khramenko NI. [Method for diagnosing disorders of accommodation based on pupillographic studies of pupillary response]. Arkhiv klinicheskoi i eksperimentalnoi meditsiny. 2001;10(2);132-3. Russian. 19.Glanz S. [Biomedical statistics]. Moscow: Praktika;1998. Russian. 20.Zaitsev VM, Lifliandskii VG, Maeinkin VI. [Tutorial in applied medical statistics]. St Peterburg:Foliant;2008. Russian. 21.Kasthurirangan S, Glasser A. Age related changes in the characteristics of the near pupil response. Vision Res. 2006 Apr;46(8-9):1393-403. 22.Kasthurirangan S, Glasser А. Characteristics of pupil responses during far-to-near and near-to-far accommodation. Ophthal Physiol Opt. 2005 Jul;25(4):328-39. 23.Math?t S. Pupillometry: Psychology, Physiology, and Function. J Cogn. 2018 Feb 21;1(1):16. 24.Adhikari P, Pearson CA, Anderson AM, et al. Effect of age and refractive error on the melanopsin mediated post-illumination response (PIPR). Sci Rep. 2015 Dec 1;5:17610. 25.Hammond CJ, Snieder H, Spector TD, et al. Factors affecting pupil size after dilatation: the twin eye study. Br J Ophthalmol. 2000 Oct;84(10):1173-6. 26.Telek HH, Erdol H, Turk A. The Effects of Age on Pupil Diameter at Different Light Amplitudes. Beyoglu Eye J. 2018;3(2):80-5. 27.Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010 May;51(5):2764-9. 28.Kardon R. Regulation of light through the pupil. In: Levin LA, Nilsson SFE, Ver Hoeve J, Wu SM, editors. Adler’s Physiology of the Eye. New York: Elsevier; 2011. pp. 502–525. 29.Tekin K, Sekeroglu MA, Kiziltoprak H, et al. Static and dynamic pupillometry data of healthy individuals. Clin Exp Optom. 2018 Sep;101(5):659-665. 30.Volkova EM. [Effect of accommodative nervous system tone on accommodation function in myopia]. Abstract of Cand Sc (Med) Thesis. Yaroslavl: Yaroslavl State Medical Academy;2007. Russian. 31.McDougal DH, Gamlin PDR. Pupillary control pathways. In: Allan IB, Akimichi K, Gordon MS, Gerald W, editors. The Senses: A Comprehensive Reference. New York: Academic Press; 2008. pp. 521–536. 32.Gamlin PDR. Neural mechanisms for the control of vergence eye movements. Ann N Y Acad Sci. 2002 Apr;956:264-72.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|