J.ophthalmol.(Ukraine).2020;4:14-22.
|
http://doi.org/10.31288/oftalmolzh202041322 Received: 27 May 2020; Published on-line: 27 August 2020 Findings of ocular and brain hemodynamics in patients with anterior uveitis complicated by macular edema N.I. Khramenko, Cand Sc (Med), N.V. Konovalova, Dr Sc (Med) SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE: Khramenko NI, Konovalova NV. Findings of ocular and brain hemodynamics in patients with anterior uveitis complicated by macular edema. J.ophthalmol.(Ukraine).2020;4:14-22.http://doi.org/10.31288/oftalmolzh202041322 Background: Uveitis is the fifth most common cause of visual loss in the developed world, accounting for about 10% of legal blindness. Macular edema (ME) has been found to be the most important cause of both blindness and visual impairment (29% and 41%, respectively) in patients with uveitis. The rate of macular edema in patients with anterior uveitis may be as high as one-third. Molecular mechanisms and pathophysiology of inflammatory retinal detachment in uveitis have been only partially elucidated. It is still unclear why some patients exhibit only one episode of ME, while others have a chronic or recurrent course of ME and are resistant to immunomodulatory and anti-inflammatory therapy. Purpose: To identify the features of ocular and brain hemodynamics in patients with anterior uveitis complicated by ME. Material and Methods: Totally, 155 uveitis patients (primary anterior uveitis, 30 patients; recurrent anterior uveitis, 125 patients) who were examined and treated at Uveitis Department and Visual Function Research Laboratory were involved in this study. Not only routine studies, but also optical coherence tomography, electrical impedance studies (ophthalmic rheography and rheoencephalography), and adaptation potential studies were performed. Results: Most cases (73.3%) with primary uveitis had a unilateral disease (p = 0.05), and as much as 50% of patients with recurrent uveitis had a bilateral disease. Macular edema was found in 18.4% of cases with primary uveitis, 20.8% of cases with frequently recurring uveitis, 3.3% of cases with infrequently recurring uveitis, and 11.5% of cases with non-identified recurrence pattern of uveitis. The rate of ME was higher in a bilateral primary disease (37.5%) than in a unilateral primary disease (p = 0.009). Macular edema was found 17% more frequently (p = 0.03) in frequently recurring uveitis than in infrequently recurring uveitis. Among patients with ME, those with frequent recurrences of anterior uveitis had the lowest mean visual acuity (0.28 ± 0.07). Compared to patients without ME, those with ME exhibited a 50% and 22.2% higher ocular pulse blood filling (OPBF, expressed as RQ, ‰ rheographic coefficient) in primary anterior uveitis and in recurrent anterior uveitis, respectively. In addition, compared to patients with uncomplicated anterior uveitis, those with ME exhibited a 71% and a 33.3% higher ocular blood flow velocity in primary anterior uveitis and in recurrent anterior uveitis, respectively. Relative pulse blood filling of the internal carotid system in patients with recurrent anterior uveitis complicated by ME was 30% higher than in patients with uncomplicated recurrent anterior uveitis. Our study of the adaptation potential, an integrative indicator of the cardiovascular system’s response to the stimulus, found that, among the anterior uveitis patients, the vast majority (84%) showed stressed adaptation mechanisms. Conclusion: Macular edema was found in 18.4% of cases with primary uveitis, 20.8% of cases with frequently recurring uveitis, and 3.3% of cases with infrequently recurring uveitis. The disease bilaterality in primary anterior uveitis and frequent recurrences in recurrent anterior uveitis were found to be aggravating factors for the development of ME. Compared to patients with uncomplicated anterior uveitis, those with ME exhibited a 50% and 22.2% higher OPBF expressed as RQ in primary anterior uveitis and in recurrent anterior uveitis, respectively. Relative pulse blood filling of the internal carotid system in patients with recurring anterior uveitis complicated by ME was 30% higher than in patients with recurring uncomplicated anterior uveitis. Keywords: anterior uveitis, complications, macular edema, ocular and brain hemodynamics, ophthalmic rheography, rheoencephalography
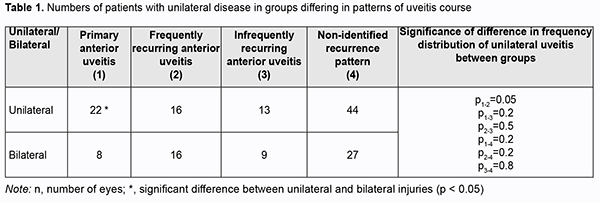
Introduction Uveitis is the fifth most common cause of visual loss in the developed world, accounting for about 10% of legal blindness [1]. The incidence and prevalence of uveitis differs based on age, anatomic location of the inflammatory process (anterior, intermediate, posterior uveitis, panuveitis), gender, histopathology (granulomatous, non-granulomatous), type of inflammatory process (acute, chronic, recurrent), and etiology (infectious, non-infectious, neoplastic or traumatic). In addition, the prevalence differs among the geographical regions [2]. Idiopathic anterior uveitis (AU) is the most common form of uveitis in the community [2]. Anterior uveitis accounts for 90% of all cases of uveitis in primary care settings and 50–60% in tertiary center settings. HLA-B27 AU is the most common type of non-infectious uveitis in most of the developed countries (except Japan and Italy) [2]. Infectious causes are common (30-60%) in the developing countries. Herpes and toxoplasmosis are the leading infectious causes of uveitis. An increase in the prevalence of infectious etiologies, including tuberculosis and syphilis, has been seen in developed countries [2]. Macular edema (ME) is the most common complication causing visual loss in patients with uveitis [3], and may occur either as a direct consequence of the disease or as a sequel of therapy [4]. In one large study from a tertiary uveitis center, ME accounted for 41% of visual impairment and 29% of blindness [5]. ME may persist despite adequate control of uveitis activity, and frequently leads to loss of central visual acuity [6]. The improved availability of imaging modalities has allowed for earlier and more accurate diagnosis of ME. Studies vary in the rate of macular edema in patients with uveitis, reported as low as 9% and as high as 70%. The rate depends on the primary anatomical site of in?ammation, imaging techniques used, and resolution of imaging instruments used. Optical coherence tomography (OCT) (90.4%) more frequently returned usable information regarding macular edema than fluorescein angiography (FA) (77%) or biomicroscopy (76%) [7], and is regarded as a gold standard for diagnosing ME in uveitis patients. The percentage incidence of ME by sites of anatomical location of uveitis was 9-28% for anterior uveitis, 40-70% for intermediate uveitis, and as much as 34% for posterior uveitis and 65% for panuveitis [3, 8]. Of note is that, although intermediate uveitis is the least frequent anatomical diagnosis of uveitis (as much as 15%), the percentage incidence of ME for patients with intermediate uveitis is rather high (as much as 70%) [3]. Three patterns of uveitic ME are observed, either isolated or in combination, cystoid ME, diffuse ME and serous retinal detachment (SRD), and a study by Rothova found cystoid ME to be the most common of these three patterns [4]. Others detected cystoid ME only in 30-50%, diffuse ME only in 37.5-50%, and SRD only in 10% of eyes with uveitic ME, with the rest attributed to a combination of SRD with either cystoid or diffuse ME [9]. In addition, epiretinal membrane was detected by OCT in 25-40%, and vitreomacular traction, in 5-10% of eyes with uveitic ME [9, 10]. The breakdown of the inner and/or outer blood retinal barriers (BRB) is the major cause of uveitic ME, and is caused by chronic inflammation and the sum-up of cytotoxic and vasogenic effects due to the immunological aggression. Extracellular fluid is accumulated either in the intraretinal or the subretinal space [11]. Molecular mechanisms and pathophysiology of inflammatory retinal detachment in uveitis have been only partially elucidated. The same is true for the factors determining whether vision will be completely restored or irreversibly lost in a particular patient. It is still unclear why some patients exhibit only one episode of ME, while others have a chronic or recurrent course of ME and are resistant to immunomodulatory and anti-inflammatory therapy. The involvement of BRB components, retinal microvascular bed and choroidal capillaries, in the pathogenesis of macular edema has been reported. Previously, we have reported on the ocular hemodynamics in eyes with anterior uveitis on the basis of ophthalmic rheography and OCT [12, 13, 14, 15]. Studies on hemodynamic impairments are important to prevent these impairments in uveitis complicated by ME. The purpose of the study was to identify the features of ocular and brain hemodynamics in patients with anterior uveitis complicated by macular edema. Material and Methods One hundred and fifty five uveitis patients (mean age, 38.6 ± 0.4 years) who were examined and treated at Uveitis Department and Visual Function Research Laboratory were involved in the study. Of these, 30 had primary anterior uveitis (38 eyes; 22 patients with unilateral uveitis and 8 patients with bilateral uveitis). The mean self-reported disease duration was 26±5 fays (range, 5 to 90 days). Patients were monitored for 90 to 180 days of the quiet period after a single episode of clinical manifestations. Anterior uveitis was classified as infectious, non-infectious, or of unknown etiology. In addition, it was defined as frequently recurring if a patient had more than one recurrent episode a year. Sixteen patients with unilateral disease and 16 with a bilateral disease (totally, 48 eyes) had frequently recurring anterior uveitis with a mean disease duration of 2090 ± 417 days. Thirteen patients with unilateral disease and 9 with a bilateral disease (totally, 31 eyes) had infrequently recurring anterior uveitis (not more than one recurrent episode a year) with a mean disease duration of 2440 ± 328 days. Recurrence data were unavailable, and uveitis recurrence pattern was not identified for 44 patients with unilateral disease and 27 with a bilateral disease (totally, 98 eyes), with a mean disease duration of 2379 ± 273 days. Patients underwent assessment of visual acuity, ophthalmoscopy, biomicroscopy, perimetry, intraocular pressure (IOP) and systemic blood pressure measurements, examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance. In addition, they underwent electrical impedance studies (ophthalmic rheography (ORG) and rheoencephalography (REG)) with Reocom, the computerized rheography apparatus. ORG included measurements of ocular pulse blood filling (OPBF, expressed as RQ, ‰ rheographic coefficient) and vascular tone (expressed as alpha/T percentage index), whereas REG included assessment of relative pulse blood filling (expressed as relative rheographic index RI, i.e., ratio of the patient’s rheographic index to that of age-matched normals, taken as 100%) of brain vessels. The level of adaptation potential (AP) was calculated according to the formula developed by Baevsky [16]: AP = 0.011*HR + 0.014*SBP + 0.008*DBP + 0.09*M – 0.009*H + + 0.014*A – 0.27 where HR is heart rate per minute; SBP is systolic blood pressure, mmHg; DBP is diastolic blood pressure, mmHg; A is age, years; M is body mass, kg; and H is height, cm. The AP was considered satisfactory (with high or adequate functional capabilities) if the AP level was < 2.1. Adaptation mechanisms were considered to be subjected to some stress (with adequate functional capabilities ensured by functional reserves) if the AP level was within 2.11–3.2. The AP was considered unsatisfactory (with reduced functional capabilities) if the AP level was within 3.21–4.3. Finally, adaptation mechanisms were considered to be failed (with abrupt loss of functional capabilities) if the AP level was > 4.31. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Data are presented as mean ± standard deviation (SD). Analysis of frequency characteristics was performed using the 2x2 contingency table for computing the chi-square score. A pairwise comparison was performed by using the paired Wilcoxon signed-rank test. Results The analysis of the groups of patients with anterior uveitis was performed while taking into account whether the disease was unilateral or bilateral, which in turn characterized the severity of the process (Table 1).
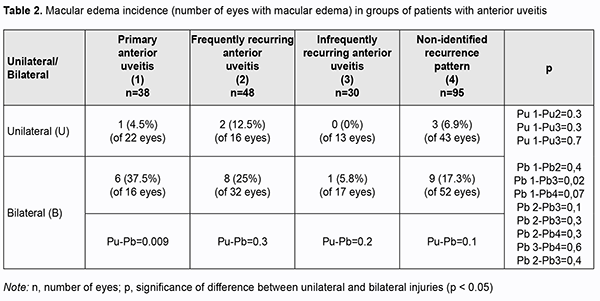
Most cases (73.3%) with primary uveitis had a unilateral disease (p = 0.05). A bilateral disease was more frequently found in patients with recurrent uveitis (42% to 50%) than in patients with primary uveitis (p > 0.05). Macular edema was found in 18.4% of cases with primary uveitis, 20.8% of cases with frequently recurring uveitis, 3.3% of cases with infrequently recurring uveitis, and 11.5% of cases with non-identified recurrence pattern of uveitis (Table 2). The rate of ME was higher in a bilateral primary disease (37.5%) than in a unilateral primary disease (p = 0.009). Macular edema was found 17% more frequently (p = 0.03) in frequently recurring uveitis than in infrequently recurring uveitis. There was no significant difference in the rate of ME for other comparisons (Table 2).
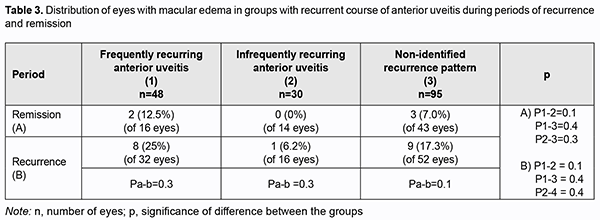
Therefore, bilaterality of uveitis in the primary process and frequent recurrences in the recurrent process are aggravating factors for the development of ME. There was no significant difference in the rate of ME among groups with recurrent uveitis (Table 3) both during recurrence and during remission. In addition, there was no significant difference in the rate of ME between patients with primary uveitis and those with recurrent disease.
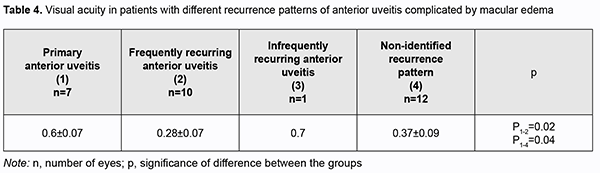
Among eyes with ME, mean visual acuity was 0.28 ± 0.07 for those with frequent recurrences of anterior uveitis, and 0.37±0.09 for those with non-identified recurrence pattern of uveitis. Compared to eyes with aggravated ME, those with primary disease process or with infrequent recurrences of anterior uveitis showed better visual acuity (0.6-0.7; p < 0.05), likely because damage to the blood retinal barrier and sensory retina was not yet severe (Table 4).
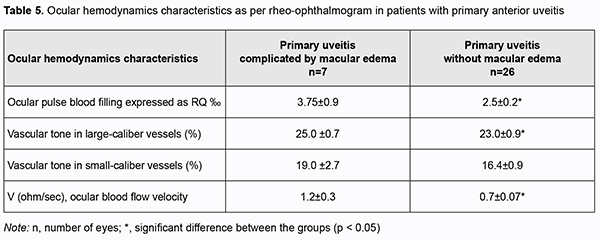
Hemodynamics is an important factor in any inflammation, because the cardiovascular system is involved in this typical physiological process. We assessed ocular hemodynamics on the basis of ophthalmic rheography in all groups of patients. Compared to eyes with uncomplicated primary uveitis, those with primary uveitis and ME exhibited a 50% higher OPBF expressed as RQ (3.75 ± 0.9‰ vs 2.5 ± 0.2‰; p = 0.03), and a 71% higher ocular blood flow velocity (1.2 ± 0.3 Ohm/s vs 0.7 ± 0.07 Ohm/s) (Table 5).
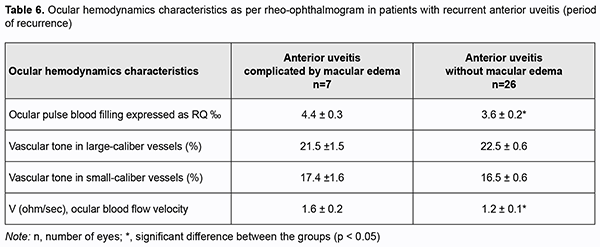
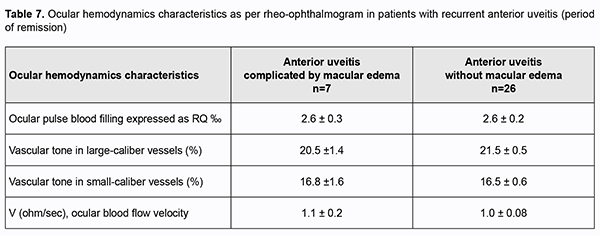
In addition, among patients with a recurrent anterior uveitis, those with ME exhibited a 22.2% higher OPBF expressed as RQ (4.4 ± 0.3 ‰ vs 3.6 ± 0.2 ‰; p < 0.05; Table 6) and a 33.3% higher ocular blood flow velocity (p < 0.05) than those with uncomplicated anterior uveitis, although no substantial difference in the vascular tone was found.Among patients with remission of recurrent uveitis, there was no difference in parameters of ocular hemodynamics between those with ME and those with uncomplicated anterior uveitis (Table 6).
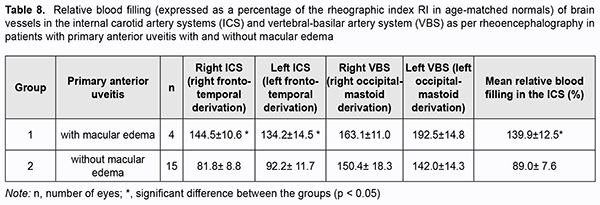
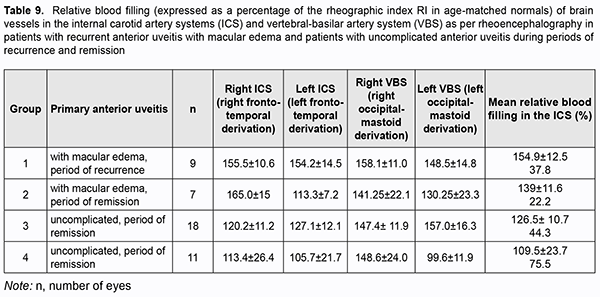
Because there were differences in the reactivity of the ocular vascular system between uveitic eyes with ME and eyes with uncomplicated anterior uveitis, and taking into account common anatomical characteristics of the blood supply systems of the anterior brain regions and the eye, we assessed pulse blood filling of the internal carotid system (ICS) and vertebral-basilar system (VBS). Pulse blood filling of the ICS in patients with primary uveitis was 11% lower than in normal individuals (p < 0.05). In patients with primary uveitis complicated by ME, pulse blood filling of the ICS was 40% higher than in normal individuals (p < 0.05) and 56% higher than in patients with uncomplicated primary uveitis (p < 0.05) ( Table 7).
Pulse blood filling of the anterior brain regions in patients with remission of recurrent anterior uveitis was not different from normal individuals, whereas that in patients with recurrent anterior uveitis was 26.5% higher than in normal individuals, but the difference was not significant (Table 8).
Pulse blood filling of the anterior brain regions in patients with recurrent anterior uveitis complicated by ME was 54% higher (p < 0.05), whereas that in patients with remission of recurrent anterior uveitis complicated by ME, 39% higher (p < 0.05) than in normal individuals. There was no significant difference in pulse blood filling of the anterior brain regions between patients with recurrent anterior uveitis complicated by ME and patients with remission of recurrent anterior uveitis complicated by ME. Compared to normal individuals, relative pulse blood filling of the ICS in patients with primary uveitis complicated by ME was 47% higher, whereas that in patients with uncomplicated primary uveitis, 17.5% higher. Relative pulse blood filling of the ICS in patients with recurring anterior uveitis complicated by ME was 30% higher than in patients with uncomplicated recurring anterior uveitis (p = 0.03; Table 9).Therefore, the results of these studies allowed us to identify the features of reactivity of the vascular systems of the brain and the eye in anterior uveitis.
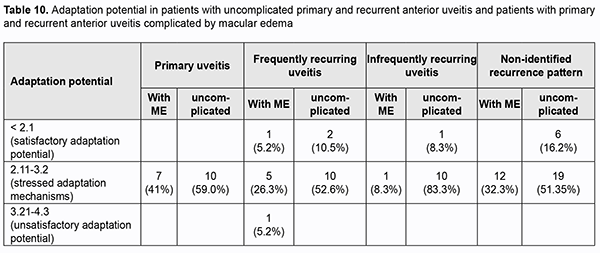
We assessed the adaptation potential as an integrative indicator of the cardiovascular system’s response to the stimulus which reflects balanced interaction of the central and peripheral mechanisms of the system. Each primary anterior uveitis patient showed stressed adaptation mechanisms with the adaptation potential within the range of 2.11 to 3.2 units (Table 10). Of the patients with recurrent anterior uveitis, 14% showed a satisfactory adaptation potential (less than 2.11 units), and 86%, stressed adaptation mechanisms. Anterior uveitis patients with ME tended to show a satisfactory adaptation potential more frequently than patients with uncomplicated anterior uveitis (Table 10). Therefore, of the anterior uveitis patients, the vast majority (84%) showed stressed adaptation mechanisms, and no one showed a severely damaged adaptation potential of the cardiovascular system’s response to the stimulus.
Discussion The rate of ME in anterior uveitis has been reported to vary from 9% to 28%, and, in recent decades, this severe complication of the disease has been paid vast attention to by the ophthalmological community. Patient age and age at the onset of chronic uveitis had been reported to be risk factors for the development of ME, and patients older than 50 years have 3.8 times higher risks for ME than younger adult patients, whereas children rarely develop ME. However, a study by Matas and colleagues [17] found no significant association with favorable prognosis of uveitic ME (as sustained improvement of both central subfoveal thickness and BCVA within a 12-month period) and demographic items including gender and age. In the current study, we found no correlation between the duration of the disease and the potential for developing ME. In addition, ME was found in 18.4% of patients with primary uveitis (with the disease duration ? 90 days). Moreover, it is important that the rate of ME was significantly higher in a bilateral primary disease (37.5%) than in a unilateral primary disease (p = 0.009), which was likely due increased inflammatory activity. However, the rate was not significantly higher in bilateral recurrent disease than in a unilateral recurrent disease. The mean age of patients with primary uveitis was 38 ± 2 years (i.e., these were individuals of the working ages), with no significant difference between patients with ME and patients with uncomplicated anterior uveitis. The mean age of patients with recurrent uveitis without ME was 37 ± 1 years, and of patients with ME, 34 ± 2 years. Therefore, there was no significant association between patient age and ME among our patient groups. We found a higher number of recurrences per year to be a significant factor for ME, which, to the best of our knowledge, has not been reported previously. There was a 17% higher ME rate (p = 0.03) in patients with more than one recurrent episode per year than in those with infrequent recurrences. The breakdown of the blood retinal barrier is the primary cause of ME in uveitis. The retinal and choroidal vasculature is a component of both the inner and outer blood retinal barriers, whereas vascular response in the focus of inflammation is an essential part of a typical inflammatory response and has the following phases: vasospasm, arterial and venous hyperemia, and stasis. Given these facts, we examined ocular hemodynamics in macular edema. Compared to patients without ME, those with ME exhibited a 50% and 22.2% higher OPBF expressed as RQ for the primary anterior uveitis group and the recurrent anterior uveitis group, respectively. In addition, compared to patients with uncomplicated anterior uveitis, uveitic patients with ME exhibited a 71% and a 33.3% higher ocular blood flow velocity for the primary anterior uveitis group and the recurrent anterior uveitis group, respectively. Previously, we have reported both on changes in OCT-based parameters of the vascular morphometry of the eye and on functional hemodynamic response in complicated anterior uveitis and activation of the sympathetic autonomic nervous system [12,14]. In this study, we have expanded on our previous work to address differences in hemodynamics of not only the eye, but also of the brain vasculature, between primary versus recurrent anterior uveitis and between patients with frequent versus infrequent recurrences. We found that the vascular brain response generally corresponded to the ocular hemodynamic response: compared to patients with uncomplicated recurrent uveitis, uveitic patients with ME exhibited a 30% higher pulse blood filling of the ICS, irrespective of whether recurrence or remission of disease was present. Our study of the adaptation potential, an integrative indicator of the cardiovascular system’s response to the stimulus, found that, among the anterior uveitis patients, the vast majority (84%) showed stressed adaptation mechanisms, and those with ME tended to show a satisfactory adaptation potential more frequently than those without ME. Conclusion First, macular edema was found in 18.4% of cases with primary anterior uveitis, 20.8% of cases with frequently recurring anterior uveitis, and 3.3% of cases with infrequently recurring anterior uveitis. Second, the disease bilaterality in primary anterior uveitis and frequent recurrences in recurrent anterior uveitis were found to be aggravating factors for the development of ME. Third, compared to patients with uncomplicated anterior uveitis, uveitic patients with ME exhibited a 50% and 22.2% higher OPBF expressed as RQ in primary anterior uveitis and in recurrent anterior uveitis, respectively. Finally, relative pulse blood filling of the internal carotid system in patients with recurring anterior uveitis complicated by ME was 30% higher than in patients with uncomplicated recurring anterior uveitis.
References 1. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004 Mar;111(3):491-500; discussion 500. 2. Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C., Androudi S. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018;26(1):2-16. 3. Rothova A, Suttorp-van Schulten MS, Treffers WF, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. Br J Ophthalmol. 1996 Apr;80(4):332-6. 4. Ad?n A, Moll-Udina A, Alba-Linero C, Figueroa-Vercellino JP, Lloren? V. Recent progress in the treatment of uveitic macular edema. Expert Rev Ophthalmol. 2019;14(4-5):227-36. 5. Levin MH, Pistilli M, Daniel E, Gangaputra SS, Nussenblatt RB, Rosenbaum JT, et al. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology. 2014 Feb. 121(2):588-95.e1. 6. Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002 Dec;10(4):239-46. 7. Kempen JH, Sugar EA, Jaffe GJ, Acharya NR, Dunn JP, Elner SG, et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology. 2013 Sep;120(9):1852-9. 8. Koronis S, Stavrakas P, Balidis M, Kozeis N, Tranos PG. Update in treatment of uveitic macular edema. Drug Des Devel Ther. 2019 Feb 19;13:667-680. 9. Tran TH, de Smet MD, Bodaghi B, Fardeau C, Cassoux N, Lehoang P. Uveitic macular oedema: correlation between optical coherence tomography patterns with visual acuity and fluorescein angiography. Br J Ophthalmol. 2008 Jul;92(7):922-7. 10. Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. 2004 May;111(5):946-53. 11. Massa H, Pipis SY, Adewoyin T, Vergados A, Patra S, Panos GD. Macular edema associated with non-infectious uveitis: pathophysiology, etiology, prevalence, impact and management challenges. Clin Ophthalmol. 2019 Sep 10;13:1761-1777. 12. Khramenko NI. Ocular hemodynamics and activity of autonomic nervous system at different stages of the course of anterior uveitis. J Ophthalmol (Ukraine). 2015;5:25-29. 13. Khramenko NI, Konovalova NV, Kravchenko LI. [Regional hemodynamics at different stages of the course of anterior uveitis]. In: [Filatov Memorial Lectures: Proceedings of the International Ophthalmological Conference]. May 24-25, 2015; Odesa. p. 118-9. Russian. 14. Khramenko NI, Konovalova NV, Shaibi Abderrakhim. [OCT study of the sensory retina and choroid in patients with uveitis]. Oftalmol Zh. 2014;3:34-40. Russian. 15. Khramenko NI, Konovalova NV, Naritsyna NI, Ivanitska OV, Serebrina TM, Kushnir VL, Rybalko AV. [Ocular hemodynamics in the complicated course of chronic recurrent anterior uveitis]. Odeskii Medychyi Zhurnal. 2016; 2(154):53-7. Ukrainian. 16. Baevskii RM. [Prediction of states on the verge of norm and pathology]. Moscow: Meditsina; 1979. pp. 248-77. Russian. 17. Matas J, Lloren? V, Fonollosa A, Esquinas C, Diaz-Valle D, Berasategui B, et al. Predictors for functional and anatomic outcomes in macular edema secondary to non-infectious uveitis. PloS one. 2019 Jan 24;14(1):e0210799.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|