J.ophthalmol.(Ukraine).2020;4:56-61.
|
http://doi.org/10.31288/oftalmolzh202045661 Received: 13 March 2020; Published on-line: 27 August 2020 Assessing in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked polyurethane composite with immobilized albucid N.A. Galatenko1, D.V. Kuliesh1, L.F. Narazhaiko1, V.P. Grytsenko1, T.Iu. Zakashun1, A.P. Maletskyy2, N.M. Bigun3 1 Institute for Chemistry of High-Molecular Compounds, Academy of Science of Ukraine; Kyiv (Ukraine) 2 SI "Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) 3 Lviv Regional Clinical Hospital; Lviv (Ukraine) E-mail: maletskiy@filatov.com.ua TO CITE THIS ARTICLE: Galatenko NA, Kuliesh DV, Narazhaiko LF, Grytsenko VP, Zakashun TIu, Maletskyy AP, Bigun NM. Assessing in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked polyurethane composite with immobilized albucid. J.ophthalmol.(Ukraine).2020;4:56-61. http://doi.org/10.31288/oftalmolzh202045661
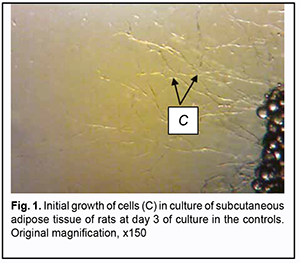
Background: Because the prevalence of ocular trauma in Ukraine remains high, restorative and reconstructive surgeries in the orbit, adnexa, and periorbital area are important; their success, however, depends on the quality of implant materials. Previously, we have developed a polymer material made of cross-linked polyurethane (PU) and containing a biologically active substance, albucid; it seems to be a promising implant material. Purpose: To assess in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked PU composites containing and not containing albucid. Material and Methods: Cell culture methodology was applied to assess in vitro cytotoxicity of cross-linked PU composites containing and not containing albucid. Subcutaneous adipose tissue of albino Wistar rats was used as a source of cells to induce growth of fibroblasts and fibroblast-like cells under culture conditions. The pH value of water solutions was determined through the study of extracts of samples of PU composites containing and not containing albucid. The extracts were examined for pH using the general-purpose pH meter EV-74 supplied with glass pH electrodes. Conclusion: It was demonstrated that fibroblast culture in Carrel flasks with controls as well as in those with extracts of samples of PU composites containing and not containing albucid was in a stable growth phase, indicating no toxic effect of the extracts on cultured cells. Polyurethane composites containing albucid were found to have a neutral pH value, which was within the acceptable range defined by hygiene standards. Keywords: cross-linked polyurethane, albucid, implant, reconstructive surgery on the eye, cytotoxicity, pH value Introduction In recent decades, there has been a tendency to the increasing prevalence of ocular trauma in Ukraine. This is mostly caused by anthropogenic and criminal-related ocular and orbital injuries, intraocular malignancies, and combat eye injuries characterized by substantial damage to ocular tissues and socket, which are frequently concomitant with traumas to face and other body parts [1, 2]. Given the above tendency, restorative and reconstructive surgeries in the orbit, adnexa, and periorbital area are important, and, if successful, they can facilitate medical, social and professional rehabilitation of patients. The ocular surgeon has to use implant materials to replace soft tissue and bone structures during restorative and reconstructive surgeries. Presently, a variety of biological [3, 4] and synthetic [5-7] implant materials are available. Biointegrable implants have an advantage of encouraging ingrowth of recipient’s tissue cells with reliable implant placement. A promising material for this purpose is a polymer material based on cross-linked polyurethane [8, 9] which contains the urethane groups (-NHCOO-) in the polymer chain, and the group is structurally close to the peptide group of proteins –СОNH–, which facilitates effective use of this class of synthetic materials. Previously, we have immobilized a biologically active substance, sulfacyl natrium (albucid), to provide biological activity to the polymer material, which may be crucial for polymer implant biointegration into pathologically altered tissue. Out previous experimental study [10] has demonstrated that the albucid-containing polyurethane implantable materials obtained were biocompatible, and their placement in the bodies of experimental animals resulted in the development of cellular responses typical of aseptic inflammation. In addition, a connective tissue capsule was observed around the albucid-containing polyurethane samples, and sample implant porosity contributed to cell migration and gradual ingrowth of tissue structures into the implants. Preliminary experimental studies demonstrated that polyurethane composites containing albucid are promising implant materials. Therefore, sanitary and chemical studies and pre-clinical studies of cross-linked polyurethane composites containing albucid are warranted prior to clinical testing. The purpose of the study was to assess in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked polyurethane composites containing and not containing albucid. Material and Methods Sanitary and chemical studies and pre-clinical studies of cross-linked polyurethane composites containing albucid were conducted within the framework of the Agreement between the Institute for Chemistry of High-Molecular Compounds and the Filatov Institute of Eye Diseases and Tissue Therapy at the Testing Laboratory (Department for Medical Polymers, Institute for Chemistry of High-Molecular Compounds) certified by the National Certification Agency of Ukraine (Certificate No. 20725 issued 25 June 2019). The methodologies as per DSTU EN ISO 10993-1:2015 (EN ISO 10993-1:2009, IDT; ISO 10993-1:2009, IDT) “Biological evaluation of medical devices – Part 1” and (EN ISO 10993-5:2009, IDT; ISO 10993-5:2009, IDT), “Biological evaluation of medical devices – Part 5” were employed taking into consideration the area and method of application of the developed polymer material. At this stage of research, we assessed in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked polyurethane composites containing and not containing albucid. Cell culture methodology as per DSTU EN ISO 10993-5:2015 (EN ISO 10993-5:2009, IDT; ISO 10993-5:2009, IDT) “Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity” was applied to assess in vitro cytotoxicity of cross-linked polyurethane composites containing albucid. Cell cultures are model test systems that allow for rapid toxicity assessment with high sensitivity and reproducibility and reliable quality control during experiments. Subcutaneous adipose tissue of albino Wistar rats was used as a source of cells to induce growth of fibroblasts and fibroblast-like cells. Carrel flask cultures containing explants of this tissue in a plasma clot medium were prepared to study cell cultures. Three groups were studied: group 1 (controls), group 2 (extracts of PU composites containing no albucid) and group 3 (extracts of PU composites containing albucid). In the control group, a small piece of tissue was embedded in a plasma clot, and the medium 199 was replaced with the fresh medium at 3, 7, 10, and 14 days. In groups 2 and 3, the medium 199 was replaced with extracts of PU composites containing no albucid, and extracts of PU composites containing albucid, respectively, at 3, 7, 10, and 14 days. The medium 199 was used a model medium for tissue cultures. The extracts were prepared using a ratio of sample weight and model media volume of 100:1 mg/mL. Extraction time was 24 hours and extraction temperature was set to 37 °C. An Amplival light microscope (Carl Zeiss, Jena, Germany) was used to observe growth of the compact zone, mesh-like zone, and migration fibroblast zone at 3, 7, 10, and 14 days. The criterion for distinguishing them was spacing between growing fibroblast cells. Sites of densely spaced growing fibroblast cells were classified as the compact zone. Sites of branched, anastomosing cords of cells were classified as the mesh-like zone. A migration fibroblast zone was defined by apices of discrete cords of cells ingrown into the solid phase of the growth medium at discrete cell locations. Extracts of samples of PU composites containing no albucid, and extracts of samples of PU composites containing albucid were examined to determine their pH values using the pH meter EV-74 supplied with glass pH electrodes. Measurement results were calculated using the following formula: ?pH = рНo-рНr, where ?pH is a change in pH value, рНо is the pH value of extract, рНr is the pH value of reference solution. The pH results were expressed as the mean of three measurements (confidence interval, 0.95). Results 1. In vitro cytotoxicity study On day 1 of culture, the first signs of growth were observed in the control group, with migration of isolated elongated cells and isolated spindle-shaped fibroblasts (Fig. 1).
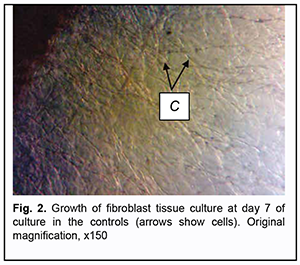
Subsequently, fibroblast growth activity increased. On day 5, growth areas in Carrel flasks were represented substantially by two types of zones: mesh-like zone (composed of cell bundles and chords of cells arranged in a mesh-like pattern), and migration fibroblast zone (composed of spindle-shaped and polygonal fibroblasts). On day 7 of culture, growth areas in Carrel flasks of the control group were represented by the compact zone (composed of spindle-shaped and polygonal cells), mesh-like zone and the zone of isolated migrating cells. The largest number of cells undergoing cycling was observed in the third zone (Fig. 2).
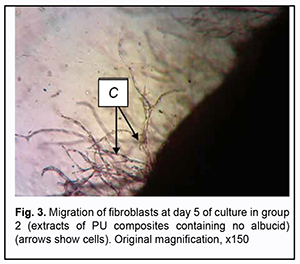
On day 10 of culture, there was an increase in growth areas in Carrel flasks of the control group, but there were signs of degenerative changes in fibroblasts of the compact zone and mesh-like zone. On day 14 of culture, the cells in Carrel flasks of the control group reached a phase of severe degeneration. As opposed to the control group, immediately after extracts of PU composites containing no albucid were added to Carrel flasks, there was some delay in migration of fibroblasts. On day 5, a balance in cell growth rates between the study group and the control group was achieved (Fig. 3).
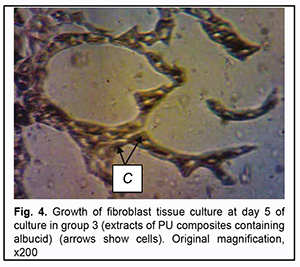
As opposed to the control group, after extracts of PU composites containing albucid were added to Carrel flasks, growth zones were represented by larger and irregularly shaped fibroblasts. Numerous isolated polygonal fibroblasts were noted at some distance from explants (Fig. 4).
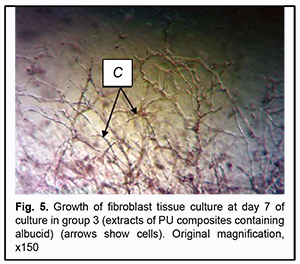
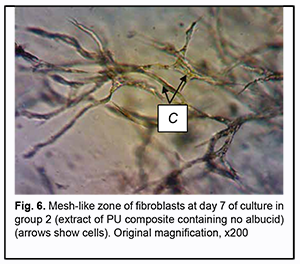
On day 7 of culture, in Carrel flasks with extracts of PU composites containing no albucid and those with extracts of PU composites containing albucid, growth areas were represented by the compact zone (composed of spindle-shaped and polygonal cells), mesh-like zone and the zone of isolated migrating cells (composed of spindle-shaped and polygonal cells). The growth zones were not smaller in area compared to those from Carrel flasks with control samples (Figs 5 and 6).
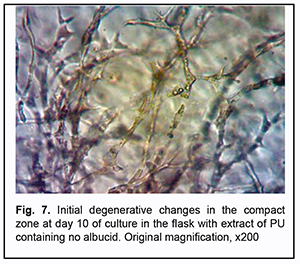
On day 10 of culture, in Carrel flasks with extracts of PU composites containing no albucid, areas of the compact zone, mesh-like growth zone and migration fibroblast zone were increased compared to controls. Initial degenerative changes (vacuolated cells, round-shaped cells) were more apparent in the compact and mesh-like growth zones in Carrel flasks with extracts of PU composites containing no albucid as well as in controls (Fig. 7).
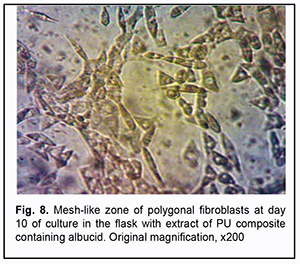
On day 10 of culture, in Carrel flasks with extracts of PU composites containing albucid, signs of degenerative changes were observed only in the compact zone. It is noteworthy that cell growth zones were wider compared to controls. Sites of growth of polygonal fibroblasts were seen in the mesh-like zone (Fig. 8). There was an increase in the area of the zone of isolated migrating cells.
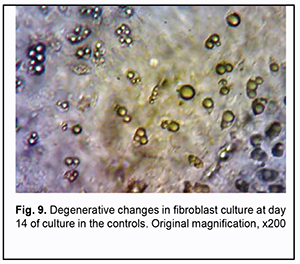
On day 14 of culture, the cells in Carrel flasks of the control group (Fig. 9) and those in Carrel flasks with extracts of PU composites containing no albucid reached a phase of degeneration, appearing round with complete loss of intercellular contacts.
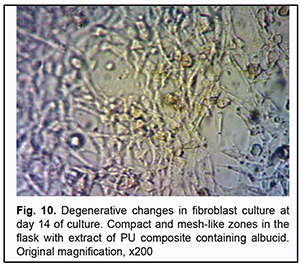
On day 14 of culture, we observed not only the cells that reached a phase of degeneration in the compact and mesh-like zones but also polygonal fibroblasts without degenerative changes (Fig. 10) in Carrel flasks with extracts of PU composites containing albucid.
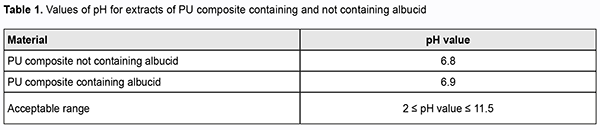
After day 21 of culture, complete cell degeneration was observed in all flasks of the control and study groups. Therefore, our cytotoxic study of PU composites containing albucid demonstrated that fibroblast culture in Carrel flasks with controls as well as in those with extracts of study samples was in a stable growth phase, indicating no toxic effect on cultured cells. 2. Determining the pH of aqueous solutions Values of рНo and рНr were each calculated as the mean of three separate measurements (Table 1).
Therefore, pH values of study materials (PU composites containing no albucid and those containing albucid) were normal and within the acceptable range defined by hygiene standards. Conclusion We conducted a study to assess in vitro cytotoxicity and pH of extracts of synthetic polymers made of cross-linked polyurethane composites containing and not containing albucid). It was demonstrated that fibroblast culture in Carrel flasks with controls as well as in those with extracts of study samples was in a stable growth phase, indicating no toxic effect of polyurethane composites on cultured cells. In addition, polyurethane composites containing a biologically active substance, albucid, were found to have a neutral pH value, which was within the acceptable range defined by hygiene standards.
References 1.Krasnovid TA. [Ocular trauma under present conditions. Providing urgent care in Ukraine]. Proceedings of the Conference of Ophthalmologists of Chernihiv, Kyiv, and other regions. Chernihiv; 2013. pp. 40-4. Russian. 2.Tselomudryi AI, Venger GE, Pogorelyi DN, Rizvaniuk AV. [Current system of stage-by-stage treatment for combat-related eye injuries in the area of ATO]. Visnyk morskoi meditsyny. 2016;2(71):196-203. Russian. 3.Grusha IaO, Fedorov AA, Dzemeshkevich VV, Blinova IV. [The clinical-and-morphological specificity of using xenopericardium in plasty of the eyelid and orbit]. Vestn Oftalmol. 2004 Sep-Oct;120(5):19-21. Russian. 4.Karaian AS. [One-stage repair of traumatic defects and deformations of the cheekbone, nose, and orbit complex]. [Dr Sc (Med) Dissertation]. Moscow: Central Research Institute for Dentistry and Maxillofacial Surgery; 2015. Russian. 5.Astakhov YuS, Nikolaenko VP, D’yakov VE. [Use of polytetrafluoroethylene implants in ophthalmic surgery]. St Petersburg: Foliant; 2007. Russian. 6.Maletskyy AP, Dubkova VI, Maievskaia OI, Bigun NM. [Results of the use of polymer composite Ikvoban in reconstructive surgery of the orbit and oculo-orbital region]. Oftalmologiia. Vostochnaia Evropa. 2013;special issue:188-97. Russian. 7.Chao DL, Harbour JW. Hydroxyapatite versus polyethylene orbital implants for patients undergoing enucleation for uveal melanoma. Can J Ophthalmol. 2015 Apr;50(2):151-4. 8.Galatenko NA, Rozhnova RA. [Biologically active polymeric materials for medicine]. Kyiv: Naukova Dumka; 2013. Russian. 9.Rozhnova RA, Shilov VV, Galatenko NA. [Structural and morphological studies of biologically active implants with prolonged healing action]. Kompozitsiini polymerni materialy. 2000;22 (2): 146-50. Russian 10.Galatenko NA, Kulyesh DV, Maletskyy AP, Karpenko OS. Soft-tissue response to synthetic polymer implants made of cross-linked polyurethane and containing a biologically active substance, albucid or dacarbazine, in animals. J. Ophthalmol. (Ukraine). 2018; 6:52–8. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|