J.ophthalmol.(Ukraine).2021;1:50-54.
|
http://doi.org/10.31288/oftalmolzh202115054 Received: 21 January 2020; Published on-line: 12 February 2021 Сomparision of retinal nerve fibre layer defects in glaucomatous and normal fellow eyes of glaucomatous patients: an OCT based study Garg P., Raj P., Rathore S. Era’s Lucknow Medical College and Hospital; Lucknow, Uttar Pradesh; (India) E-mail: drpriyankarajy@gmail.com TO CITE THIS ARTICLE: Garg P, Raj P, Rathore S. Сomparision of retinal nerve fibre layer defects in glaucomatous and normal fellow eyes of glaucomatous patients: an OCT based study. J.ophthalmol.(Ukraine).2021;1:50-4. http://doi.org/10.31288/oftalmolzh202115054 Purpose. To determine the concordance of retinal nerve fiber layer (RNFL) thickness in glaucomatous and normal fellow eyes and also to compare it with normal individuals using optical coherence tomography (OCT). Material & method. An observational cross-sectional study including 73 primary open angle glaucoma cases and 73 normal individuals not having primary open angle glaucoma (POAG) was done. RNFL thickness of both eyes was measured using OCT by fast RNFL thickness protocol. Average RNFL thickness, quadrantic that is inferior, superior, nasal and temporal, and sectoral RNFL thickness was evaluated. The values were compared among the fellow normal eyes and glaucomatous eyes of the same patient and also with the eyes of individuals not having glaucoma. Results. The average RNFL thickness for normal eyes was 90.65±15.04 μm, glaucomatous eyes was 76.83 ± 14.02 μm and fellow eyes of glaucomatous patients was 82.06±14.60 μm. A statistically significant difference was seen both between non glaucomatous and glaucomatous group and also between glaucomatous and fellow eyes of glaucomatous group. (p<0.001) Conclusion. Symmetry of RNFL defects was seen between glaucomatous patients and their fellow eyes corresponding with the pattern of early glaucomatous damage. Key words: RNFL, glaucoma, nerve fiber layer, OCT, optical coherence tomography, visual field
Introduction. Glaucoma is characterised by loss of ganglion cells and their axons, the Retinal Nerve Fiber Layer (RNFL). The loss of retinal ganglion cells in glaucoma is irreversible, therefore early detection is critical to prevent progression of the disease (by 1-4 yrs). Studies have shown that RNFL defects occur prior to visual field loss [1, 2] and also prior to Optic Nerve Head (ONH) changes [3, 4]. Also, it was found that RNFL evaluation is more sensitive for predicting future visual field loss compared to ONH evaluation and that the RNFL is a better predictor of damage than cup-disc ratio (CDR) [5, 6]. For visual field defects to be evident on white on white perimetry, it requires at least 30-40% of neuronal cell loss to occur Therefore, RNFL assessment has emerged as an important parameter for pre-perimetric diagnosis of glaucoma and this is done quite accurately by OCT machines by which it is possible to detect the loss of retinal nerve fiber layer Even though glaucoma is a bilateral disease, the structural and functional damage is asymmetric between the two eyes. Approximately 7-25% of glaucoma patients with unilateral visual field loss at baseline will later experience visual field damage in the fellow eyes and thus a more sensitive RNFL analysis by OCT could be an early indicator for impending damage caused by POAG [7]. Materials and methods This is a hospital based cross sectional study carried out at a tertiary care center of north India for the purpose of which 146 individuals including 73 healthy volunteers without POAG and 73 patients of POAG were recruited after patient’s consent and institutional ethical clearance adhering to the tenets of Declaration of Helsinki. All participants underwent detailed ophthalmological examination. Uncorrected and best corrected visual acuity assessment by Snellen’s chart, slit lamp examination, gonioscopy, tonometry by applanation tonometer, fundus examination by direct and indirect ophthalmoscope and by + 90-D lens, standard automated perimetry by Humphrey perimeter, and retinal nerve fiber layer analysis by optical coherence tomography by carl Zeiss OCT machine were performed. All patients below 40 years of age, having more than 4D of refractive error, any corneal or lens opacity, any other intraocular disease, any recent intraocular surgery or history of ocular trauma, having primary angle closure or secondary glaucoma, diabetes or any other neurological disorder affecting optic disc, RNFL thickness or visual fields were excluded from the study. Inclusion criteria for normal subjects was age > 40 years, best corrected visual acuity (BCVA) of 6/60 or better; less than 4D of refractive error, intraocular pressure (IOP) less than or equal to 21 mm Hg with no history of elevation of IOP in the past. Appearance of normal (healthy) optic disc, by direct and indirect ophthalmoscopy and standard automated perimetry (SAP) results within normal limits. Inclusion criteria for glaucoma group was patients with age > 40 years, IOP > 21 mm hg on three or more testing taken at different times of the day; vertical asymmetry of CDR > 0.2 between the two eyes or high CDR > 0.6 and visual field changes specific to POAG. Patients satisfying all the inclusion criteria for glaucoma group were included in the study. All participants underwent visual field testing using Humphrey Field Analyzer with either 24-2 full threshold or SITA- standard. Standard protocol of static white on white stimuli commonly known as SAP (30-2) was used to study the fields. Eyes with unreliable visual fields (false negatives >30%, false positive >30%, and fixation loses >15%) were excluded from the study. RNFL thickness measurement was done using cirrus SD-OCT (software version 4.0). Patients were dilated using 1% tropicamide before images were recorded and fast RNFL scans were performed. This protocol provides better reproducibility than a single scan. It consists of three circular scan each 3.46 mm in diameter, centred on the optic disc. Various machine generated parameters were used for evaluation of RNFL thickness including the average RNFL thickness, RNFL thickness in each quadrant- superior (46◦ to 135◦), inferior (226◦ to 315◦), nasal (136◦ to 225◦) and temporal (316◦ to 45◦) and RNFL thickness in 12 -50 degree clock hours sectors (12’o clock is superior position and 9’o clock is in the temporal position). The mean age ± standard deviation (SD) was 61 ±10.4 (range 50 to 62) with no significant difference of age and sex among the groups. There were 39.04% (57/146) males and 60.96% (89/146) females in the total study group. The range of mean deviation (MD) of glaucomatous patients on automated perimetry was -8.86 to +1.01 with mean ± standard deviation of -2.06 ± 6.80 (range= +1.01 to -8.86). The average best corrected visual acuity of the normal eyes was 6/18 and that for the glaucoma eyes was 6/24 and 6/18 for the fellow eyes The data was then collected, compiled and statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 21.0 statistical Analysis software. Quantitative data were summarized in mean ± Standard Deviation whereas Qualitative data were summarized in proportion percentages. The prevalence was shown in percentages with 95%confidance intervals. Groups were compared for quadrants and sector RNFL thickness by one-way analysis of variance (ANOVA). P value of less than 0.05 (p<0.05) was considered statistically significant. Results 146 eyes of 73 POAG patients (73 glaucomatous eyes and 73 fellow eyes of same patients) and 73 eyes of age matched normal individuals not having POAG were examined. On examining the RNFL thickness around optic nerve in glaucomatous and fellow eyes and comparing it with normal control eyes, we found a decrease trend in the RNFL thickness in glaucomatous and fellow eyes as compared to the normal control eyes. Intergroup comparison between the glaucomatous, fellow and normal eyes showed a statistically significant difference in all the quadrants as well as in overall average thickness (p<0.05) except in the temporal quadrant (Table 1).
We further assessed the sectoral RNFL thickness around the optic nerve. A decrease in RNFL thickness was seen in both the glaucomatous and the fellow eyes in all the sectors as compared to the normal controls but was statistically significant in sector 1, 2, 7, 11 and 12 only (p<0.05) (Table 2). We also compared the RNFL thickness in each sector in the fellow eye with the glaucomatous eye to see the frequency of glaucomatous defects in the fellow eyes of glaucoma patients. Sectors 1 (41%) 2 (31%), 3 (21%), 7 (59%), 11 (52%) and 12 (42%) showed maximum percentage of eyes with glaucomatous defects similar to the glaucomatous eye which was statistically significant (p= 0.001, 0.001, 0.029, 0.001, 0.001 and 0.001, respectively). Sectors with the highest agreement were sector 7 (59%) and sector 11 (52%) while sectors with the lowest agreement were sector 9 (10%) and sector 4 (20%) (Table 3).
Discussion Current study is a cross sectional analysis to evaluate the correlation in location (sectors and quadrants) of RNFL thinning between fellow eyes in glaucoma patients. OCT measured retinal nerve fiber layer (RNFL) thickness may detect glaucoma related damage before the development of visual field defects (VFD) [8, 9]. RNFL thickness assessment has shown high reproducibility [10] and reliability [11] following the advancements in OCT machines and softwares, thus making it an important tool in glaucoma diagnosis and management. As thinner OCT RNFL measurements have been shown to be a risk factor for the development of glaucomatous change in glaucoma suspect eyes [12], knowledge of location of the damage in the worse eye could lead to a more careful examination of corresponding RNFL thickness and visual field defects in fellow eye. In our study a significant decrease in RNFL thickness was observed in the glaucomatous eyes and also the fellow eyes of glaucomatous patients as compared to normal population in all the quadrants. Highest correspondence in RNFL thinning was seen in sector 7 and sector 11(p<0.001) of fellow and glaucomatous eyes of the cases while on looking for quadrantic correlation highest agreement on defective locations was for inferior and superior quadrants(p<0.001) which confirms the typical pattern of early glaucomatous damage. Al-Otaibi [13] in the study on 60 healthy subjects and 30 glaucomatous subjects observed that the mean value of RNFL thickness decreased with age but it was minimum in glaucomatous patients. In a study by Kim and associates [14], and Anton A et al. [15], the average RNFL thickness and superior and inferior RNFL thickness were significantly less in glaucomatous eyes than in eyes with ocular hypertension or fellow eyes of glaucomatous patients signifying subclinical RNFL damage. Subbiah et al. [16] compared RNFL thickness between normal, ocular hypertensive and glaucomatous eyes and found a statistically significant decreasing trend in the RNFL thickness. The authors concluded that structural RNFL damage is already detectable in fellow eyes of NTG, particularly in the inferior and superior sectors, which are the most commonly damaged locations of the optic nerve in glaucoma Similar to our study, Boden C et al. [17] investigated intereye concordance in location of visual field defects in POAG on standard automated perimetry. Four hundred and ninety eyes of 245 patients were examined. The superior arcuate defects (24%) and inferior partial arcuate defects (26%) had the greatest correspondence between fellow eyes with a higher correspondence in more advanced defects and hence, concluded a spatial relationship between defective locations of an individual’s eyes. Shin et al. [18] conducted a study similar to ours in normal tension glaucoma (NTG) and noticed a significant decrease in RNFL thickness in normal fellow eyes of NTG as compared to controls (p<0.001). Bertuzzi et al. [19] conducted a similar study on 77 glaucoma patients (154 eyes) and RNFL thickness at each location (12 clock-hour sectors and 4 quadrants) was measured and compared with non-glaucomatous controls. Fellow eyes showed significant correlations of RNFL thickness in all sectors and quadrants. Categorical ranking also showed significant agreement between fellow eyes in sectors 1 (P <0.01) and 7 (P <0.03), and in the superior quadrant (P <0.01), inferior quadrant (P <0.04), and total RNFL thickness (P <0.04). Liu et al. [20]. their study found that RNFL thickness loss was seen in a substantial number of the contralateral eyes of glaucoma patients showing unilateral progression by conventional methods. The control eyes in their cohort had faster rates of RNFL loss than would be expected with normal aging, losing on average 0.71 μm/year, which may support the hypothesis that some of these control eyes had actually glaucomatous progression that had not yet resulted in detectable changes by conventional methods. It can be deducted that fellow normal eyes of glaucomatous patients are at risk of glaucomatous damage as compared to normal healthy individuals, even though the changes and IOP may not be indicative of the same. Subclinical damage process does occur in normal eyes of the glaucomatous patients which may be missed on clinical examination. OCT guided evaluation of RNFL thickness may serve as a useful adjunct in accurately and more objectively diagnosing POAG even in its early stages, much before the disease becomes clinically evident and also can be helpful in predicting the regional damage in the fellow eye of unilateral glaucoma patients. 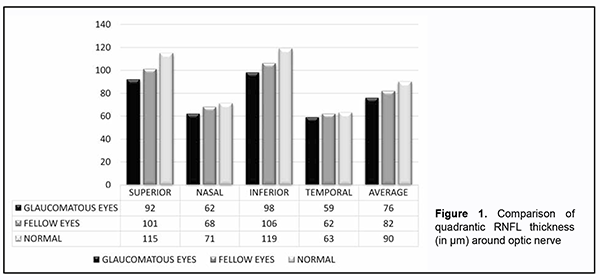 
References 1.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991 Jan;109(1):77-83. 2.Caprioli J, Prum B, Zeyen T. Comparison of methods to evaluate the optic nerve head and nerve fiber layer for glaucomatous change. Am J Ophthalmol. 1996 Jun;121(6):659-67. 3.Sommer A, Miller NR, Pollack I, Maumenee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 1977 Dec;95(12):2149-56. 4.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992 Jan;99(1):19-28. 5.Quigley HA. Examination of the retinal nerve fiber layer in the recognition of early glaucoma damage. Trans Am Ophthalmol Soc. 1986;84:920–966. 6.Zhou Q, Weinreb RN. Individualized compensation of anterior segment birefringence during scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2221-8. 7.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004 Nov;82(11):844-51. Epub 2004 Dec 14. 8.Mwanza JC, Budenz DL. New developments in optical coherence tomography imaging for glaucoma. Curr Opin Ophthalmol. 2018 Mar;29(2):121-129 9.Sakata LM1, Deleon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve - a review. Clin Exp Ophthalmol. 2009 Jan;37(1):90-9. 10.Schuman JS, Pedut-Kloizman T, Hertzmark E, Hee MR, Wilkins JR, Coker JG, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996 Nov;103(11):1889-98. 11.Carpineto P, Ciancaglini M, Zuppardi E, Falconio G, Doronzo E, Mastropasqua L. Reliability of nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology. 2003 Jan;110(1):190-5. 12.Lalezary M, Medeiros FA, Weinreb RN, Bowd C, Sample PA, Tavares IM, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006 Oct;142(4):576-82. 13.Al-Otaibi BS. Retinal Nerve Fiber Layer Thickness between Normal Eyes & Glaucoma. Nig J Med Rehab. 2015 Jun 21;18(1) 14.Kim DM, Hwang US, Park KH, Kim SH. Retinal nerve fiber layer thickness in the fellow eyes of normal-tension glaucoma patients with unilateral visual field defect. Am J Ophthalmol. 2005 Jul;140(1):165-6. 15.Anton A, Moreno-Montañes J, Blázquez F, Alvarez A, Martín B, Molina B. Usefulness of optical coherence tomography parameters of the optic disc and the retinal nerve fiber layer to differentiate glaucomatous, ocular hypertensive, and normal eyes. J Glaucoma. 2007 Jan;16(1):1-8. 16.Subbiah S, Sankarnarayanan S, Thomas PA, Nelson Jesudasan C A. Comparative evaluation of optical coherence tomography in glaucomatous, ocular hypertensive and normal eyes. Indian J Ophthalmol 2007;55:283-7 17.Boden C, Hoffmann EM, Medeiros FA, Zangwill LM, Weinreb RN, Sample PA. Intereye concordance in locations of visual field defects in primary open-angle glaucoma: diagnostic innovations in glaucoma study. Ophthalmology. 2006 Jun;113(6):918-23. Epub 2006 Apr 27. 18.Shin YU, Lee SE, Cho H, Kang MH, Seong M. Analysis of peripapillary retinal vessel diameter in unilateral normal-tension glaucoma. J Ophthalmol. 2017 Jun; 1-7 19.Bertuzzi F, Hoffman DC, De Fonseka AM, Souza C, Caprioli J. Concordance of retinal nerve fiber layer defects between fellow eyes of glaucoma patients measured by optical coherence tomography. Am J Ophthalmol. 2009 Jul;148(1):148-54. 20.Liu T, Tatham AJ, Gracitelli CP, Zangwill LM, Weinreb RN, Medeiros FA. Rates of Retinal Nerve Fiber Layer Loss in Contralateral Eyes of Glaucoma Patients with Unilateral Progression by Conventional Methods. Ophthalmology. 2015 Nov;122(11):2243-51 No conflict of interest was declared by the authors
|


