J.ophthalmol.(Ukraine).2021;3:34-40.
|
http://doi.org/10.31288/oftalmolzh202133440 Received: 11 January 2021; Published on-line: 29 June 2021 In vitro investigation of the effect of photosensitizer-mediated 365-nm UV light and 630-670-nm low-energy laser irradiation on the fungal flora, Candida albicans and Fusarium spp L. F. Troichenko, G. I. Drozhzhyna, A. L. Moloda, L. V. Dolenko SI «The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: tlf2008@ukr.net TO CITE THIS ARTICLE:Troichenko LF, Drozhzhyna GI, Moloda AL, Dolenko LV. In vitro investigation of the effect of photosensitizer-mediated 365-nm UV light and 630-670-nm low-energy laser irradiation on the fungal flora, Candida albicans and Fusarium spp. J.ophthalmol.(Ukraine).2021;3:34-40. http://doi.org/10.31288/oftalmolzh202133440 Background: Infectious corneal ulcers and infectious keratitis are a major global cause of visual impairment and blindness. Although there are numerous antimicrobial agents available, novel methods should be designed to allow for fast and comprehensive microbicidal and microbistatic response on their target with minimum toxic effect to the body in order to preserve vision in patients with severe corneal infections. Purpose: To assess in vitro the antimicrobial effect of photosensitizer-mediated 365-nm ultraviolet (UV) irradiation in combination with 630-670 nm low-energy laser irradiation on the suspensions of Candida albicans and Fusarium spp. Material and Methods: The Mueller-Hinton medium was used to conduct a routine disc diffusion susceptibility test and assess the antimicrobial activity of the preparations. Methods for exerting effect on test strains of Candida albicans and Fusarium spp isolated from the conjunctival sac: The method of low-energy laser irradiation (clinically, photodynamic therapy or PDT) was as follows. A sterile disc was placed, along with test strains of microorganisms, on the surface of the medium. Methylene blue 0.1% was instilled on the surface of the sterile disc until the disc was completely covered. Thereafter, the disc was irradiated with 630-670-nm low-energy laser for three minutes. The method of UV irradiation (clinically, collagen cross-linking or CXL) was as follows. The sterile disc was placed, along with test strains of microorganisms, on the surface of the medium. Riboflavin 0.1% was instilled on the surface of the sterile disc until the disc was completely covered. Thereafter, the disc was irradiated with 365-nm UV light delivered by the UVX 2000 for 10 minutes. Results: Growth inhibition zone analysis found that Candida albicans was susceptible to PDT as well as to CXL. The diameter of the growth inhibition zone after treatment with PDT plus CXL plus fluconazole was significantly, 6.3 mm, larger than for the control disc with fluconazole. Fusarium spp was found to be susceptible to PDT plus CXL as well as to PDT plus CXL plus itraconazole, with the diameter of the growth inhibition zone being significantly, 4.2 mm and 7.8 mm, respectively, larger than for the control disc with itraconazole. Conclusion: In the in vitro experiment, the combination treatment (365-nm UV light using riboflavin 0.1% as a photosensitizer and 630-670-nm low-energy laser irradiation using methylene blue 0.1% as a photosensitizer) we proposed had a demonstrated antimicrobial effect on Candida albicans and Fusarium spp, showing fungal growth inhibition. This experimental study showed that the approach is promising and warrants further research in ophthalmology. Keywords: ultraviolet irradiation, low-energy laser irradiation, antimicrobial effect, Candida albicans, Fusarium spp Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
Introduction Corneal infections can have a profound effect on visual function. Infectious corneal ulcers and infectious keratitis are a major global cause of visual impairment and blindness [1-3]. Although there are numerous antimicrobial agents available, severe corneal infections require skilled management and effective chemotherapy to preserve vision [3,4]. If diagnosis and initiation of appropriate antimicrobial treatment are delayed, it has been estimated that only 50% of the eyes heal with a good visual outcome [5]. The proportion of keratitis cases attributed to fungi has been reported from as low as 4.5% to as high as 50% (Behrens-Baumann W.1999, Said.G.2011). In a large case series of cases with fungal keratitis reported from 11 tertiary eye care centers across the United States, in addition to contact lens wear (37%) and ocular trauma, ocular surface disease was the third most common risk factor accounting for 29% of cases. Fungal keratitis most commonly arises from yeasts (such as Candida alb., Malassezia spp., Micosporum spp.) and molds (such as Aspergillus spp., Cephalsporium spp., Mucor spp., Fusarium spp., Paecillomices spp.), with the latter causing more severe ocular lesions, which potentially lead to loss of the eye [1, 6-8]. Inadequate topical and long-term systemic treatment for fungal keratitis may induce severe complications. For decades, antibiotics (particularly, fluoroquinolones) have been used extensively in the treatments for various diseases. Concerns that this practice has contributed to increased bacterial resistance to antimicrobials have led to developing the novel treatment techniques that can provide fast and comprehensive microbicidal response on their target with minimum toxic effect to the body [1]. Riboflavin, or vitamin B2, is a naturally occurring compound and an essential human nutrient. Japanese scientists demonstrated in the 1960s that riboflavin, when exposed to visible or UV light, could be used to inactivate the RNA containing tobacco mosaic virus. Research has been underway since 2000 in using riboflavin as a photosensitizer to inactivate pathogens in plasma, platelet and red cell products [7-14]. Studies have demonstrated that riboflavin can act as a photosensitizer useful for the inactivation of pathogens found in corneal infections, because of its nucleic acid specificity and its limited tendency toward indiscriminate oxidation. On the other hand, the antimicrobial activity of ultraviolet (UV) irradiation includes sporicidal and virucidal effects [15-17]. Corneal collagen cross-linking (CXL) was established as a gold standard for the treatment of corneal collagen cross-linking (CXL) at the World ophthalmology congress in Vienna in 2011. Photochemical ionization takes place and riboflavin is destroyed (with release of free oxygen) by exposure to UV-A radiation generated by the UV-X system. Free oxygen-derived radicals cause cross-linking between -СН and –CN groups in collagen molecules, which induces their binding to form a 3D meshwork. Numerous additional bounds between corneal collagen fibers result in a significant improvement of corneal mechanical strength and rigidity. Biomechanical studies have shown that corneal rigidity increases by 350%-380% after cross-linking. An increase in corneal mechanical strength and rigidity begins immediately after CXL, and continues during two years [9, 18]. The clinical use of a combination of riboflavin and UV for CXL and the observations in the laboratory of keratocyte depletion and apoptosis after its application, stimulated researchers to use CXL for corneal infection. CXL has been shown to have antimicrobial, antienzimatic and anti-inflammatory effects, to result in an increase in strength of the stroma, and to improve corneal resistance to degradation by microbial enzymes. The antimicrobial effect is most important and results from the application of ultraviolet-A irradiation to a riboflavin-soaked cornea [14]. An experimental study by Martins and colleagues (2008) [15] demonstrated that Riboflavin/UVA was effective against Pseudomonas aeruginosa (PA), Staphylococcus aureus (SA), Staphylococcus epidermidis (SE), and Streptococcus pneumoniae (SP), but was ineffective on Candida albicans (CA). Although most researchers agree that CXL has a profound effect in the treatment of bacterial keratitis and bacterial ulcers, there is substantial disagreement on the efficacy of CXL in the treatment of fungal and Acanthamoeba keratitis [15-31]. Since the literature is scarce on the use of CXL for the treatment of keratitis and corneal ulcers of mixed etiology, developing novel combination treatments for these severe corneal disorders is important. Antimicrobial photodynamic therapy (PDT) with methylene blue as photosensitizer has been used as adjunct treatment against ocular bacterial and fungal keratitis since 2012 [6, 32, 34]. The efficacy of PDT using a laser with a 639-nm to 670-nm wavelength and methylene blue 0.1% has been demonstrated in a model of fungal keratitis as well as a model of acute bacterial endophthalmitis. The proposed technique has been found clinically effective in the treatment of patients with severe fungal keratitis, with fungicidal effect as early as week 2 of treatment and subsequent longitudinal improvements in clinical characteristics [6, 32, 34]. The treatment effect of the combination of CXL with subsequent methylene blue PDT is based on the UV-induced damage to the DNA and RNA of microorganisms and fungicidal and bactericidal effect of PDT. It is noteworthy that previously published reports on the efficacy of CXL for the treatment of infectious keratitis include isolated cases which differ from each other in etiology, and depth and severity of corneal lesions. Therefore, it is important to perform experimental in vitro and in vivo studies on CXL (using riboflavin 0.1%) in combination with methylene blue PDT using a laser with a 639-nm to 670-nm wavelength. The purpose of this study was to assess in vitro the antimicrobial effect of 365-nm UV irradiation using riboflavin 0.1% as a photosensitizer in combination with 630-670 nm low-energy laser irradiation using methylene blue 0.1% as a photosensitizer on the fungal flora, Candida albicans and Fusarium spp. Material and Methods The Mueller-Hinton medium was used to conduct a routine disc diffusion susceptibility test and assess the antimicrobial activity of the preparations as per the national guidelines MV9.95-143-2007. Concentrated Mueller-Hinton medium was prepared according to the instructions of manufacturer (Farmaktiv LLC, Kyiv, Ukraine) and poured into Petri dishes. Cultures of the organisms (Candida albicans (CA) and Fusarium spp (FS)) from the patient’s conjunctival sac were used as test strains. All the cultures were adjusted to 0.5 McFarland standard, which is visually comparable to a microbial suspension of approximately 1.5 × 108 cfu/cm3. The optical density of the suspension was measured with an optical density meter. An aliquot of 1 to 2 cm3 of the standardized inoculum was pipetted out onto the surface of the Petri dish containing the medium. After the Petri dish was opened, it was allowed to dry at room temperature for 10-15 minutes. Sterile disc, fluconazole disc, and itraconazole disc were applied to the medium surface by using sterile forceps. Methods for exerting effect on test strains of Candida albicans and Fusarium spp isolated from the conjunctival sac The method of low-energy laser irradiation (clinically, photodynamic therapy) was as follows. A sterile disc was placed, along with test strains of microorganisms, on the surface of the medium. Methylene blue 0.1% was instilled on the surface of the sterile disc until the disc was completely covered. Thereafter, the disc was irradiated with 630-670-nm low-energy laser for three minutes. The method of UV irradiation (clinically, collagen cross-linking or CXL) was as follows. The sterile disc was placed, along with test strains of microorganisms, on the surface of the medium. Riboflavin 0.1% was instilled on the surface of the sterile disc until the disc was completely covered. Thereafter, the disc was irradiated with 365-nm UV light delivered by the UVX 2000 for 10 minutes. Experimental Methods included the following methods for exerting effect on test strains of Candida albicans and Fusarium spp isolated from the conjunctival sac: Photodynamic therapy (PDT), Collagen cross-linking (CXL) PDT plus CXL, and PDT plus CXL plus either fluconazole (for Candida albicans) or itraconazole (for Fusarium spp.) The fluconazole disc and itraconazole disc were used as control discs for Candida albicans and Fusarium spp, respectively, and placed on the surface of the medium in the Petri dish at a distance of at least 5 cm from the experimental disc. Immediately after performing experiments and applying discs, the Petri dishes were incubated in the upside down position at 35° C for 24 hours. Recording of results After incubation, the dishes were placed upside down on a black diffusing surface, with the light falling on them at an angle of 45 degrees above the horizontal (the records were made using the reflected light setting). The antipathogenic activity was assessed in the dishes based on the diameters of the growth inhibition zone surrounding the discs. The diameters of growth inhibition zones were measured in millimeters with the help of a scale (Figs 1, 2).
Statistical analyses were conducted using Statistica 9.0 (StatSoft, Tulsa, OK, USA) software. Student t-test was used to determine the significance of differences in mean values. Results Candida albicans was found to be susceptible to PDT as well as to CXL. The diameter of the growth inhibition zone after treatment with PDT only ranged from 15 mm to 28 mm (М 21.9± SD 4.7 mm), which was 7.7 mm smaller than for the fluconazole control disc (range, 28 mm to 30 mm; М 29.6± SD 0.69 mm) (р =0.001). In addition, the diameter of the growth inhibition zone after treatment with CXL only ranged from 0 mm to 19 mm (М 9.1± SD 9.6 mm), which was 22 mm smaller than for the fluconazole control disc (range, 30 mm to 34 mm (М 31.1± SD 1.37 mm), and the difference was statistically significant (Table 1).
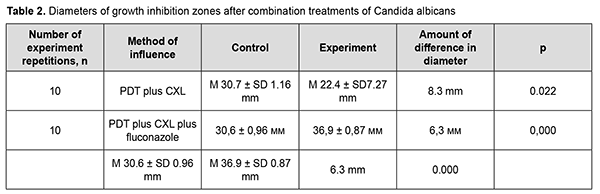
Moreover, the diameter of the growth inhibition zone after treatment with PDT plus CXL ranged from 14 mm to 30 mm (М 22.4± SD 7.27 mm), which was 8.3 mm smaller than for the fluconazole control disc (М 30.7± SD 1.16 mm; range, 29 mm to 32 mm). The largest diameter of the growth inhibition zone was observed after treatment with PDT plus CXL plus fluconazole (range, 36 mm to 38 mm; М 36.9± SD 0.87 mm), which was 6.3 mm larger than for the control disc with fluconazole (range, 29 mm to 32 mm; М 30.6± SD 0.96 mm) (Table 2).
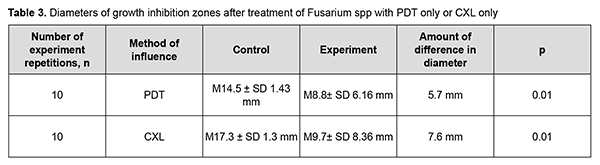
The following results were obtained after experiments with Fusarium spp. The diameter of the growth inhibition zone after treatment with PDT ranged from 0 mm to 14 mm (М 8.8± SD 6.16 mm), which was significantly smaller than for the control disc with itraconazole (range, 13 mm to 17 mm (М 14.5± SD 1.43 mm) (р =0.01). In addition, the diameter of the growth inhibition zone after Fusarium spp treatment with CXL ranged from 0 mm to 17 mm (М 9.7± SD 8.36 mm), which was significantly smaller than for the control disc with itraconazole (range, 15 mm to 19 mm (М 17.3± SD 1.3 mm) (р =0.01) (Table 3).
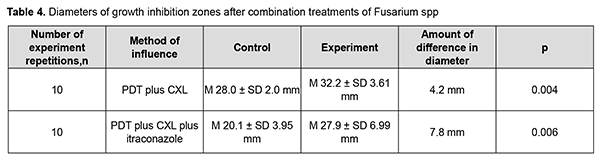
Moreover, the diameter of the growth inhibition zone after Fusarium spp treatment with PDT plus CXL ranged from 25 mm to 35 mm (М 32.2± SD 3.61 mm), which was 4.2 mm larger than for the control disc with itraconazole (range, 24 mm to 28 mm (М 28.0± SD 2.0 mm) (р =0.004). The diameter of the growth inhibition zone after Fusarium spp treatment with PDT plus CXL plus itraconazole was 27.9± SD 6.99 mm (range, 20 mm to 58 mm), compared to 20.1± SD 3.95 mm (range, 17 mm to 31 mm) for the control disc with itraconazole (Table 4, Fig. 3).
Discussion Growth inhibition zones were found after Candida albicans treatment with PDT only, as well as with PDT plus CXL plus fluconazole. In addition, growth inhibition zones were found after Fusarium spp. treatment with PDT plus CXL, and with PDT plus CXL plus itraconazole. We proposed an in vitro combination treatment for fungal infections of the cornea, because we have failed to find in the literature a definite scheme of treatment for this severe disorder, fungal lesions in the cornea. Said and colleagues (2014) [31] demonstrated that PACK-CXL may be an effective adjuvant therapy in the management of severe infectious keratitis (particularly, fungal keratitis) associated with corneal melting. In a study by Shetty and colleagues [27], three of six patients with fungal keratitis resolved following CXL treatment, but patients with deep stromal keratitis or endothelial plaque failed to resolve. Saglk and colleagues (2013) [28] presented a case of fungal corneal ulcer unresponsive to medical treatment, successfully treated with the use of UV-A and riboflavin CXL administered twice with an interval of three weeks. In a study by Arboleda and colleagues (2014) [29], the isolates recovered from patients with confirmed fungal keratitis were used in the experiments, and rose bengal-mediated PDT successfully inhibited the growth of 3 types of fungi. In has been demonstrated experimentally and clinically by Zborovska [6, 32, 34] that methylene blue mediated PDT was effective in the treatment of Candida albicans-induced keratitis. Recent literature reviews [5, 30] on the diagnosis and treatment of fungal keratitis concluded that management of the disease remains a challenge to cornea specialists. Emerging fungal pathogens and resistance to existing antifungal drugs have further added to the reasons for poor prognosis in fungal keratitis [5, 30]. Newer antifungal agents and newer methods of targeted drug delivery system can be helpful in treating recalcitrant cases. Nanoparticles and antimicrobial peptides have shown promise in experimental studies and offer hope for improving prognosis in cases of fungal keratitis in future. In the in vitro experiment, the combination treatment (365-nm UV light using riboflavin as a photosensitizer and 630-670-nm low-energy laser irradiation using methylene blue 0.1% as a photosensitizer) we proposed had a demonstrated antimicrobial effect on Candida albicans and Fusarium spp, showing fungal growth inhibition zones. This experimental study demonstrated that the approach is promising and warrants further research in ophthalmology. References 1.Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013 May 29;3(1):47. 2.Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010 Dec;117(12):2263-7. 3.Garg P. Fungal, mycobacterial, and nocardia infections and the eye: An update. Eye (Lond). 2012 Feb; 26(2): 245–51. 4.Neroev VV, Petukhova AB, Danilova DYu, et al. [The cross-linking of corneal collagen in treatment of trophic and bacterial ulcers of cornea]. Rossiiskii meditsinskii zhurnal. 2013;(2):25-8. Russian. 5.Maharana PK, Sharma N, Nagpal R, et al. Recent advances in diagnosis and management of Mycotic Keratitis. Indian J Ophthalmol. 2016 May;64(5):346-57. 6.Zborovska AV, Gorianova IS, Kuriliuk AN. [Efficacy of methylene blue photodynamic therapy with 630-670-nm low-energy laser irradiation in the treatment of patients with fungal keratitis]. Oftalmol Zh. 2012;4:12-5. Russian.Crossref 7.Spoerl E, Wollensak G, Dittert D, Seiler T. Thermomechanical behavior of collagen-cross-linked porcine cornea. Ophthalmologica. Mar-Apr 2004;218(2):136-40. 8.Kasparova EA, Yang B, Bocharova YA, Novikov IA. [Application of visible longwave radiation for inactivation of microorganisms]. Vestn Oftalmol. 2020;136(6):42-9. doi: 10.17116/oftalma202013606142. Russian. 9.Hafezi F, Randleman J, eds. Corneal Collagen Cross‐Linking. Thorofare, NJ: Slack Inc.; 2013:43‐104. 10.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010 Oct;23(4):884-928. 11.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29(1):35–40. 12.Chan E, Snibson GR, Sullivan L. Treatment of infectious keratitis with riboflavin and ultraviolet-A irradiation. J Cataract Refract Surg. 2014 Nov;40(11):1919-25. 13.Tschopp M, Stary J, Frueh BE, Thormann W, Bocxlaer V, Tappeiner C. Impact of Corneal Cross-linking on Drug Penetration in an Ex Vivo Porcine Eye Model. Cornea. 2012 Mar;31(3):222-6. 14.Stewart M, Lee OT, Wong FF, Schultz DS, Lamy R.Cross-Linking with Ultraviolet-A and Riboflavin Reduces Corneal Permeability. Invest Ophthalmol Vis Sci. 2011 Nov 29;52(12):9275-8. 15.Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3402-8. 16.Schrier A, Greebel G, Attia H, Trokel S, Smith EF. In vitro antimicrobial efficacy of riboflavin and ultraviolet light on Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa. J Refract Surg. 2009 Sep;25(9):S799-802. 17.Sauer A, Letscher-Bru V, Speeg-Schatz C, Touboul D, Colin J, Candolfi E, Bourcier T. In vitro efficacy of antifungal treatment using riboflavin/UV-A (365 nm) combination and amphotericin B. Invest Ophthalmol Vis Sci. 2010 Aug;51(8):3950-3. 18.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA—Riboflavin Cross-Linking of the Cornea. Cornea. 2007;26(4):385–9. 19.Galperin G, Berra M, Tau J, Boscaro G, Zarate J, Berra A. Treatment of fungal keratitis from Fusarium infection by corneal cross-linking. Cornea. 2012 Feb;31(2):176-80. 20.Ferrari T, Leozappa M, Lorusso M, Epifani E, Ferrari L. Keratitis treated with ultraviolet A/riboflavin corneal cross-linking: a case report. Eur J Ophthalmol Mar-Apr 2009;19(2):295-7. 21.Al-Sabai N, Koppen C, Tassignon MJ. UVA/riboflavin crosslinking as treatment for corneal melting. Bull Soc Belge Ophtalmol. 2010;(315):13-7. 22.Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts / Iseli HP. Cornea. 2008 Jun;27(5):590-4. 23.Ehlers N, Hjortdal J. Riboflavin-ultraviolet light induced cross-linking in endothelial decompensation. Acta Ophthalmol. 2008 Aug;86(5):549-51. 24.Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010 Dec;29(12):1353-8. 25.Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultraviolet lightmediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97(5):669–671. 26.Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UV-A riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2012 Jan;250(1):95-102. 27.Shety R, Nagaraja H, Jayadev C, Shivanna Y, Kugar T. Collagen crosslinking in the management of advanced non resolving 28.microbial keratitis. Br J Ophthalmol. 2014 Aug;98(8):1033-5. 29.Saglk A, Uçakhan OO, Kanpolat A. Ultraviolet A and riboflavin therapy as an adjunct in corneal ulcer refractory to medical treatment. Eye Contact Lens. - 2013 Nov;39(6):413-5. 30.Arboleda A, Miller D, Cabot F, Taneja M, Aguilar MC, Alawa K, et al. Assessment of rose bengal versus riboflavin photodynamic 31.therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014 Jul;158(1):64-70.e2. 32.Pranita Sahay, Singhal D., Nagpal R., Maharana P.K., Farid M., Gelman R, et al. Pharmacologic therapy of mycotic keratitis. Surv Ophthalmol. May-Jun 2019;64(3):380-400. 33.Said DG, Elalfy MS, Gatzioufas Z, El Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross linking with photoactivated riboflavin (PACK CXL) for the treatment of advanced infectious keratitis with corneal melting.Ophthalmology. - 2014 Jul;121(7):1377-82. 34.Zborovska AV. [Photodynamic therapy using methylene blue as a photosensitizer in the treatment of fungal keratitis]. Oftalmol Zh. 2011;2:54-8. Russian. 35.Pasyechnikova NV, Zborovska AV, Kustrin TB. [Effect of laser-activated methylene blue on Escherichia coli culture]. Oftalmol Zh. 2009;3:60-3. Russian.
|