J.ophthalmol.(Ukraine).2021;3:66-69.
|
http://doi.org/10.31288/oftalmolzh202136669 Received: 25 January 2021; Published on-line: 29 June 2021 Lens nucleus masquerading as recurrent uveal melanoma: a case report M. M. Umanets, V. O. Naumenko, T. M. Tukilush SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: filatovretina@gmail.com TO CITE THIS ARTICLE:Umanets MM, Naumenko VO, Tukilush TM. Lens nucleus masquerading as recurrent uveal melanoma: a case report. J.ophthalmol.(Ukraine).2021;3:66-69. http://doi.org/10.31288/oftalmolzh202136669 This paper presents a case of the presence of the lens nucleus on the fundus at the site of the scleral bed of the excised melanoma, which masqueraded as a recurrent tumor. Keywords: uveal melanoma, lens nucleus Conflict of Interest Statement: We declare no conflict of interest.
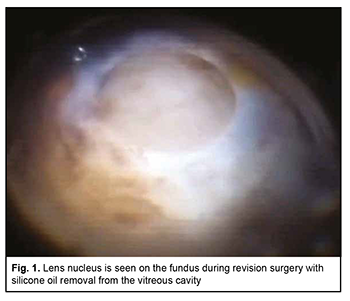
Uveal melanoma (UM) is the most common primary intraocular malignancy that arises from melanocytes within the uveal tract of the eye [1, 2]. Ophthalmoscopically, early UM appears as a prominent slate-grey or yellow-brown lesion with indistinct margins, accumulation of lipofuscin on its surface, and various levels of pigmentation. However, as the tumor progresses, it may become cupola-shaped or mushroom-shaped due to rupture of the Bruch membrane. UM more commonly presents in a postequatorial location (75%-90%) that in an equatorial location [3-7]. Currently available treatment options include eye-preserving techniques (laser coagulation, photocoagulation, thermotherapy, plaque brachytherapy, external irradiation, endoresection, exoresection and lamellar sclerouveoectomy), their combinations, and eye-removal techniques (enucleation and evisceration) [2]. The treatment option selected for the patient depends on numerous factors such as size and location of the neoplasm and the presence of signs of invasion of adjacent ocular or extraocular structures [8, 9]. Until recently, eye-preserving techniques have been limited by a mean tumor dimensions, and enucleation has been the most common technique for treating large melanomas [10]. However, the devastating psychological consequences of an eye-removal technique, patient’s refusal to undergo eye removal surgery in some cases, and long-term irradiation-induced complications (like radiation retinopathy, marked exudative response and cataract) urged researchers to look for alternative approaches to the treatment of UM. Endoresection has become such an alternative; it may be considered as a primary procedure or as a second stage after laser and/or radiotherapy [11-17]. Although this approach has a number of advantages, its utility for treatment of UM is limited by a high rate of intraoperative complications in the form of intensive bleeding. In addition, a potential problem with the technique is the intraoperative dissemination of vital tumor cells that can lead to recurrences [12, 18]. The endoresection technique features endodiathermy of the retinal vessels and endoresection with an increase in irrigation fluid pressure from to 60 mmHg to 100 mmHg for homeostatic purposes, which is insufficient for achieving complete homeostasis. We have developed a technique for endoresection of choroidal melanoma using high-frequency electric welding of biological tissues [19]. A positive local treatment outcome for choroidal melanoma will contribute to improved long-term prognosis. Local recurrence significantly increases the risk of metastatic uveal melanoma and mortality [20] and a time to local recurrence has been reported to range from one month to 9.8 years after treatment [20]. However, recurrent UM with extrabulbar extension may occur as late as 11 years after UM endoresection without adjuvant brachytherapy [21]. Hereby we present a case of a 56-year-old female patient who presented to the Filatov Institute with complaints of low vision (no pattern vision) and pain in and redness of the right eye. She believed she had been ill since March, 2018, when she was diagnosed with and underwent a combined eye-preserving treatment for a malignant choroidal tumor in the right eye, which involved brachytherapy, endoresection of choroidal melanoma using high-frequency electric welding of biological tissues, endolaser coagulation and vitreous tamponade with 5,700-centistoke silicone oil. There was no clinical or ultrasound evidence of positive tissue, the scleral bed of the excised tumor was clean, and visual acuity (VA) of the affected eye was 0.14 with a spherical correction of +6.0D to 0.17 with a spherical correction of +6.0D, VA of the fellow-eye was 1.0 during the follow-up after endoresection of choroidal melanoma. Because the right eye showed evidence of cataract progression with a decrease in uncorrected visual acuity to 0.02 during the first half of 2020, the patient was recommended to undergo ultrasound phacoemulsification of cataract with IOL implantation without silicone oil removal in this eye. In spring, 2020, she presented to the Filatov Institute with complaints of low vision and redness of the right eye. Her ocular history was significant for ultrasound phacoemulsification of cataract with IOL implantation in the right eye two weeks prior, which was performed at the patient’s regional hospital. In addition, according to the hospital discharge report, the procedure was unremarkable and there were no difficulties. At presentation, UCVA in the right eye was accurate light projection, and in the left eye, 1.0. The IOP values via Maklakoff applanation tonometry were 38 mmHg OD and 18 mmHg OS. On examination of the right eye, the eye appeared irritated, the cornea swollen, the anterior chamber of moderate depth, the pupil round and movable, a posterior chamber IOL implanted on the anterior lens capsule and dislocated and displaced anteriorly and inferiorly from the capsular bag. In addition, there was neither posterior lens capsule nor vitreous body, and the vitreous cavity appeared filled with silicone oil. Ophthalmoscopy was difficult due to corneal opacity and swelling caused by ocular hypertension, but showed a moderately pale optic disc with clear margins, narrowed retinal vessels, and a scleral bed of the excised melanoma in the inferior nasal quadrant with extension to the macula. It is noteworthy that ophthalmoscopy found a prominent optically non-transparent and grayish brown mass in the center of a surgical coloboma of the retina and choroid. The mass was round, with a diameter of six to seven optic disc diameters, and was immobile with changes in head and eye positions. Due to optic aberration of the silicone bubble (the volume of the silicone bubble in the vitreous cavity was an estimated 75%), in the inferior vitreous cavity, the tissue of the “neoplasm” seemed to gradually pass into the sclera, and the margins of the “neoplasm” were not well defined. It was difficult to determine whether this “neoplasm” was vascular or not, and to reveal the details of its structure due to the reduced transparency of the optic media. All things considered, the patient was preliminary diagnosed with recurrent uveal melanoma. On examination of the left eye, the anterior segment was unremarkable. Other findings in the left eye included mild opacity of the lens, destruction of the vitreous, pale pink optic disc, unremarkable macula, peripheral vitreochorioretinal degeneration at the 12 o’clock position and the retina appeared to be attached. Although no difficulty in ultrasound phacoemulsification in the right eye had been stated in the hospital discharge report, it was possible that the lens nucleus could migrate to the fundus, because the posterior lens capsule was absent, and the IOL had been implanted onto the anterior lens capsule. However, the “neoplasm” was immobile, located at the scleral bed of the excised choroidal melanoma, and had indistinct margins; these facts indicated that the lens nucleus hardly migrated to the fundus. It was decided to use a battery of additional imaging tests. Ultrasound of the right eye detected silicone oil at the site of the vitreous body, but no large focus of intraocular pathology. Infrared digital imaging of the right fundus with long-wave illumination [20, 22] showed a round focal mass with heterogeneous surface and well-defined margins in the posterior segment, inferiorly and temporally to the macula; the infrared light was reflected irregularly from the focal mass which appeared to adhere to the inner wall of the globe in the projection of the scleral bed of the excised tumor. The infrared signal was reflected intensely from the sclera around the focal mass. Transpalpebral near-infrared transillumination of the right eye showed to pathological shadows on the sclera in the anterior segment. Visualization of fundus structures by optical coherence tomography (OCT) of the right eye was limited due to the reduced transparency of the optic media. OCT showed an oval-shaped, low-reflective focal mass with well-shaped margins in the posterior segment, inferiorly and temporally to the macula; the mass extended into the vitreous cavity, was parietally located and shielded deeper structures. Preoperative diagnostic assessment did not allow to make a definite differential diagnosis between recurrent uveal melanoma and the presence of the lens nucleus on the fundus. The findings of ophthalmoscopy of the right eye (i.e., the location of a round, prominent, and immobile mass at the site of scleral bed of the excised melanoma; the color of the mass; the margins of the mass were not well defined; the mass was immobile with changes in head and eye positions) suggested that it was recurrent uveal melanoma. However, the shape and homogeneity of the mass, an IOL located anteriorly to the capsular bag and dislocated inferiorly, and the findings of additional imaging tests (ultrasound, infrared ophthalmoscopy, and OCT) made us suspect that there was a lens nucleus at the center of the scleral bed, and the immobility of this nucleus could be explained by the presence of silicone oil in the vitreous cavity. It was therefore decided to conduct a revision surgery with silicone oil removal from the vitreous cavity, and the patient agreed to have this surgery done. In brief, the operation field and conjunctiva were disinfected and sub-Tenon anesthesia with 2% lidocaine was used. Thereafter, a standard 23-G three-port technique was employed for active silicone oil removal. After the vitreous cavity was revised, the nucleus was found on the surface of the scleral bed (Fig. 1), and was fragmented and removed with vitreous cutter.
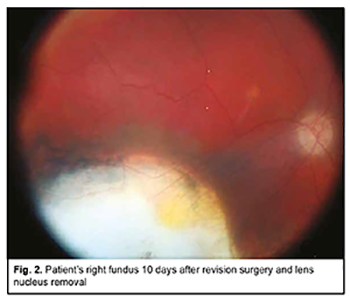
Subsequently, the IOL was repositioned, fluid-gas exchange and sterile air tamponade performed, and the sclerotomies closed with suturing. It is noteworthy that no intraoperative complication was encountered. Early postoperatively, there were signs of postoperative uveitis in the form of iris color change, posterior synechiae, and fibrin film in the papillary aperture, which were relieved by anti-inflammatory medications. The volume of the air bubble in the vitreous cavity was an estimated 80% and IOP was within a normal range. Ophthalmoscopically, the optic disc was somewhat pale, with well-defined margins, the retinal vessels narrow, the scleral bed of the excised melanoma clean, and the retina appeared to be attached. At day 7 after surgery, the patient was discharged, the volume of the air bubble in the vitreous cavity was an estimated 25%, UCVA in the right eye was accurate light projection, the iris had a normal color, and the pupil was round and reactive to light. At day 10 (at discharge), UCVA was 0.04 OD, and, ophthalmoscopically, the optical media were transparent, the retina appeared to be attached, and no signs of tumor growth were seen (Fig. 2).
All research was performed in accordance with the declaration of Helsinki. Informed consent was obtained from the patient. Therefore, we reported a case of the presence of the lens nucleus on the fundus at the site of the scleral bed of the excised melanoma after phacoemulsification, which masqueraded as a recurrent tumor. Recurrent uveal melanoma should be differentiated from the presence of the lens nucleus on the fundus at the site of the scleral bed of the excised melanoma after phacoemulsification to allow for adequate diagnosis and treatment in cases of suspected recurrent tumor. It is essential to make clinic/hospital discharge reports (epicrises) up properly, with sufficient data, since in the case presented here, the failure to make a record of intraoperative phaco complications (rupture of the posterior capsule with nucleus migration to the fundus) made diagnosis challenging. References 1.Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11: 279-89. 2.Bell DJ, Wilson MW. Choroidal melanoma: Natural history and management options. Cancer Control. Sep-Oct 2004;11(5):296-303. 3.Brovkina AF, editor. [Ophthalmic oncology]. Moscow: Meditsina; 2002. Russian. 4.Neroev VV, editor. [Ophthalmology: Clinical Recommendations]. Moscow: GEOTAR-Media; 2019. 5.Brovkina AF, Panova IE, Saakian SV. [Ophthalmic oncology: achievements over the last two decades]. Vestn Oftalmol. Nov-Dec 2014;130(6):13-9. Russian. 6.Brovkina AF, Astakhov YS. [Guidelines for Clinical Ophthalmology]. Moscow: Meditsinskoe informatsionnoe agenstvo; 2014. Russian. 7.Avetisov SE, Egorov EA, Moshetova LK, Neroev VV, Takhchidi KhP, editors. [Ophthalmology: National guidelines]. Moscow: GEOTAR-Media; 2013. Russian. 8.Damato BE, Heimann H, Kalirai H, Coupland SE. Age, survival predictors, and metastatic death in patients with choroidal melanoma: tentative evidence of a therapeutic effect on survival. JAMA Ophthalmol. 2014;135(2):605-13. 9.Margo CE. The Collaborative Ocular Melanoma Study: an overview. Cancer Control. Sep-Oct 2004;11(5):304-9. 10.García-Arumí J, Zapata MA, Balaguer O, Fonollosa A, Boixadera A,. Martinez-Castillo V. Endoresection in high posterior choroidal melanomas: long-term outcome. Br J Ophthalmol. 2008;92(8):1040-5. 11.Bornfeld N, Talies S, Anastassiou G, Schilling H, Schüler A, Horstmann GA. [Endoscopic resection of malignant melanomas of the uvea after preoperative stereotactic single dose convergence irradiation with the Leksell gamma knife]. Ophthalmologe. 2002 May;99(5):338-44. doi: 10.1007/s00347-002-0647-4. German. 12.Ferreyra HA, Goldbaum MH, Weinreb RN. Endoresection of irradiated choroidal melanoma as a treatment for intractable vitreous hemorrhage and secondary blood-induced glaucoma. Semin Ophthalmol. 2008;23(2):135-8. 13.Karkhaneh R, Chams H, Amoli FA, Riazi-Esfahani M, Ahmadabadi MN, Mansouri MR, et al. Long-term surgical outcome of posterior choroidal melanoma treated by endoresection. Retina. 2007 Sep;27(7):908-14. 14.Kertes PJ, Johnson JC, Peyman GA. Internal resection of posterior uveal melanomas. Br J Ophthalmol. 1998 Oct; 82(10):1147-53. 15.Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol. 2004 Jun;15(3):244-51. 16.Gündüz K, Bechrakis NE. Exoresection and endoresection for uveal melanoma. Middle East Afr J Ophthalmol. 2010 Jul;17(3):210-6. 17.Modarres M, Rezanejad A, Falavarjani KG. Recurrence and massive extraocular extension of choroidal malignant melanoma after vitrectomy and endoresection. Indian J Ophthalmol. 2014 Jun;62(6):731-3. 18.Foulds WS, Damato BE, Burton RL. Local resection versus enucleation in the management of choroidal melanoma. Eye (Lond). 1987;1 (Pt 6):676-9. 19.Umanets NN, Pasyechnikova NV, Naumenko VA, Maletskyi AP, Chabotarev EP, Pukhlik ES. Endoresection of choroidal melanoma using high-frequency electric welding of biological tissues. J. Ophthalmol. (Ukraine). 2016;4:11-14. 20.Pasyechnikova N, Naumenko V, Korol A, Zadorozhnyy O. Digital imaging of the fundus with long-wave illumination. Klin Oczna. 2009;111(1-3):18-20. 21.Peyman GA, Cheema RA, Lagouros PA. Endoresection of a ciliochoroidal melanoma. Can J Ophthalmol. 2001;36(7):411–4. 22.Zadorozhnyy OS, Alibet Y, Kryvoruchko A, Levytska G, Pasyechnikova N. Dimensions of ciliary body structures in various axial lengths in patients with rhegmatogenous retinal detachment. J Ophthalmol (Ukraine). 2017;6(479):32-6.
|