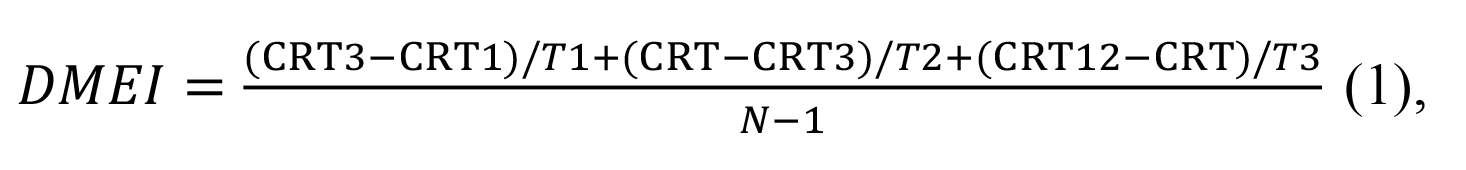J.ophthalmol.(Ukraine).2021;4:9-18.
|
http://doi.org/10.31288/oftalmolzh20214918 Received: 08 August 2021; Published on-line: 16 August 2021
Predicting DME recurrence following surgical treatment for diabetic maculopathy in a Ukrainian population of patients with type 2 diabetes mellitus Iu. O. Panchenko 1, S.Yu. Mogilevskyy 2, S.O. Rykov 2, D.G. Zhaboiedov 1, M.M. Umanets 3, S.V. Ziablitsev 1 1 Bogomolets National Medical University; Kyiv (Ukraine) 2 Shupyk National Healthcare University of Ukraine; Kyiv (Ukraine) 3 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NMAS of Ukraine"; Odesa (Ukraine) E-mail: panchenko@laserplus.com.ua TO CITE THIS ARTICLE: Panchenko IuO, Mogilevskyy SIu, Rykov SO, Zhaboiedov DG, Umanets MM, Ziablitsev SV. Predicting DME recurrence following surgical treatment for diabetic maculopathy in a Ukrainian population of patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine). 2021;4:9-18. http://doi.org/10.31288/oftalmolzh20214918 Background: Recurrent diabetic maculopathy (DMP) and diabetic macular edema (DME) may develop in the early or late period even after an advanced vitreoretinal procedure. It has been reported that the recurrence rate at one month and at one year after vitreoretinal surgery varied from 6.6% to 20.8% and from 20.8% to 83.3%, respectively. Purpose: To develop a model for predicting recurrence of DME after surgical treatment for DMP in the Ukrainian population of patients with type 2 diabetes mellitus (T2DM). Material and Methods: The study included 313 patients with T2DM (313 eyes) who underwent various types of vitreoretinal surgery. Preoperatively, enzyme-linked immunosorbent assays were used to determine blood PDGF-BB, TNFα and ЕТ1 levels, and polymorphisms of PDGFB (rs1800818) and TNFα (rs1800629) were investigated by real-time polymerase chain reaction. At months 1, 3, 6 and 12 after surgery, incidence of DME recurrence and associations of DME recurrence with the characteristics under study were assessed. Multivariate logistic regression analysis was used to develop a model in Statistica 10 (GLZ; StatSoft, Tulsa, OK, USA). Results: Index of diabetic macular edema progression (DMEI) was used as the dependent variable and calculated as the mean speed of change in central retinal thickness (CRT) over a year after the initiation of treatment for DMP. SNP rs1800818 in PDGFB, SNP rs1800629 in TNFα, blood PDGF-BB level, and CRT0 were found to be significant predictors of the risk for postoperative DME recurrence. The calculation of DMEI values allowed to divide possible genotype combinations into three groups: those promoting the regression (СС-GG; CC-GA; TC-GG), those with a stable DMEI value (TC-GA and TC-AA), and those promoting the progression of DME. Conclusion: The prediction of DME recurrence after various types of vitreoretinal surgery combined with other methods of surgical treatment for DMP in a Ukrainian population of patients with T2DM is based on the determination of gene polymorphisms of PDGFB and TNFα. Keywords: diabetic macular edema, surgical treatment, recurrences, recurrences, rs1800629, rs1800818, PDGF-ВВ, TNFα, type 2 diabetes mellitus
Introduction Diabetic maculopathy (DMP) which is severe and resistant to conservative and laser treatment, intravitreal anti-VEGF therapy and steroid therapy; DMP with epimacular membrane formation and changes in the internal limiting membrane (ILM), presence of retinal tangential traction or retinal axial traction (vitreomacular syndrome, vitreous changes, partial vitreous hemorrhage, central preretinal and subhyaloid hemorrhages); and diabetic macular edema (DME) with the presence of retinal fibrovascular membranes and risk for tractional retinal detachment, are treated with surgery which includes vitrectomy with endolaser coagulation of the retina, gas-and-air or silicone tamponade, removal of the posterior hyaloid, and, if required, ILM peeling [1-5]. However, recurrent DMP and DME may de-velop in the early or late period even after an advanced surgical procedure. It has been reported [6] that the recur-rence rate at one month and at one year after vitreoretinal surgery varied from 6.6% to 20.8% and from 20.8% to 83.3%, respectively. The DME recurrence rate after vitreoretinal intervention is performed may vary depending on the combination of methods used as well as the preoperative state of the eye [1, 3, 5, 7]. We have previously reported that a DME recurrence rate at one after surgery was 29.8% [8]. In addition, the recurrence rate depended on the stage of diabet-ic retinopathy (DR) immediately before treatment, with a maximum recurrence rate of 33.7% found for prolifera-tive DR. Moreover, the DME recurrence rate varied from 84% to 100% depending on the type of vitreoretinal sur-gery [9]. The above findings warranted the development of the system for predicting recurrence of DME after surgical treatment for DMP on the basis of biochemical markers of the pathological process [10, 11]. We believed that en-dothelial dysfunction characteristics, such as endothelin-1 (ET1), rumor necrosis factor (TNF)-α, and platelet-derived growth factor (PDGF-BB) could be used as such markers [12]. Well-known risk factors for DME also include disease duration and stage, obesity, arterial hypertension, genetic polymorphisms, etc. [13, 14]. The purpose of the study was to develop a model for predicting recurrence of DME after surgical treatment for DMP in a Ukrainian population of patients with type 2 diabetes mellitus. Material and Methods The study included 313 patients with T2DM (313 eyes) and diabetic maculopathy. These included patients with mild nonproliferative DR (NPDR; Group 1; n=40), moderate or severe NPDR (Group 2; n=92), and proliferative DR (PDR; Group 3; n=181). Patients underwent an eye examination according to the ETDRS protocol, which included visual acuity assessment, static Humphrey perimetry (Humphrey Field Analyzer (HFA II-i, Carl Zeiss Meditec, Dub-lin, CA, USA)), refractometry (RK 600, Reichert, Inc., Depew, NY), autorefractometry (Accuref-K 9001, Rexxam Co., Osaka, Japan), tonometry (Topcon CT-80, Topcon Corp, Tokyo, Japan), gonioscopy with Goldmann three-mirror lens (Volk Optical, Mentor, OH), slit-lamp biomicroscopy with SL120 and SL130 (Carl Zeiss Meditec) and Volk Super Field lens and Goldmann three-mirror lens (Volk Optical), fundus photography (the ETDRS seven standard fields) with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan) and ocular ultrasonography (Sono-med E-Z scan AB 5500, Sonomed Inc., Lake Success, NY). In addition, spectral domain optical coherence tomography (SD-OCT; Copernicus REVO, Optopol Technology Sp, zo.o, Zawiercie, Poland; scan programs, Retina 3D and Retina Raster) and OCT (Retina Angio mode) were per-formed. Fluoresecein angiography (FA) was performed with the fundus camera if (a) mild vitreoretinal neovasculariza-tion or proliferation was suspected but not identified with ophthalmoscopy or fundus photography or (b) the visual function did not correspond either to ophthalmoscopic changes in the macula or OCT findings. Severity of DR and DMP was graded as per the International Clinical Diabetic Retinopathy and Diabetic Macu-lopathy Disease Severity Scales issued by the American Academy of Ophthalmology in 2002 [1]. The DMP recur-rence that developed following vitreoretinal intervention was identified by the presence of microaneurysms, micro-hemorrhages, hard exudates in the macular area, as well as macular edema, with the latter diagnosed on the basis of an increase in central retinal thickness (CRT) to 270 µm or more. Preoperatively, ELISA was used to determine blood PDGF-BB, TNFα and ЕТ1 levels with Human PDGF-BB Quantikine ELISA Kit (R&D Systems; USA), and kits from Bender Medsystems, and Biomedica Immunoassays (Austria), respectively. Polymorphisms of PDGFB (rs1800818; locus, 22q13.1; location, intron of 5′-UTR Variant) and TNFα (rs1800629; SNP in the promoter region, 308G/A; location, 6p21.33) were investigated by real-time pol-ymerase chain reaction (PCR). At the first phase of the study, DNA extraction from venous blood was done using the Invitrogen™ PureLink Genomic DNA kit for purification of genomic DNA (Invitrogen Inc.) following the manu-facturer’s instructions. At the second phase of the study, real-time PCR was conducted using TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA) and Gene Amp® 7500 PCR System (Applied Biosystems, Fos-ter City, CA). Ninety-five age- and sex-matched individuals without ocular pathology or T2DM were used as controls. The study design and protocol were approved by the ethics committee of Shupyk National Healthcare Universi-ty of Ukraine, adhered to the tenets of the Declaration of Helsinki and European Convention on Human Rights and Biomedicine, and were compliant with the relevant legal requirements of Ukraine. Informed consent was obtained from all patients. Patients received one of the four types of surgical treatment: only 25+ three-port closed subtotal vitrectomy (CSTV; n=78); CSTV combined with internal limiting membrane (ILM) peeling (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); and CSTV combined with ILM peeling, PRLC and cata-ract phacoemulsification (phaco) (n=69). Follow-up visits were at months 1, 3, 6 and 12 after surgery. Index of diabetic macular edema progression (DMEI) was used as the dependent variable and calculated as the mean speed of change in CRT over a year after the initiation of treatment for DMP (1).
where CRT1, CRT3, CRT6 and CRT12 are CRT values at months 1, 3, 6 and 12, respectively; T1, T2, and T3, duration of the period between CRT measurements in months (T1 = 2; T2 = 3; T3 = 6); N, num-ber of CRT measurements. Multivariate logistic regression analysis was used to develop a model in Statistica 10 (GLZ; StatSoft, Tulsa, OK, USA). A set of independent variables for the development of regression equations was formed based on the princi-ple of availability and adequacy of the data that can be obtained in the initial period of patient observation at en-rollment in the study. These variables were as follows: age, gender, severity and duration of T2DM, degree of hy-perglycemia compensation as assessed by glucose and glycated hemoglobin (НbA1c) levels, DR stage and severity on the ETDRS scale, central retinal thickness (CRT0), blood levels of ЕТ1, TNFα, and PDGF-BB, and genotypes of PDGFB rs1800818 and TNFα rs1800629 (“101”, “102”, and “103” were used as dichotomous values for wild ho-mozygote, heterozygote, and minor homozygote, respectively). In the first phase of the development of multivari-ate regression models, significant predictors were selected from the set of independent variables based on the esti-mation of beta coefficients regarding the significance of their difference from the null hypothesis (p < 0.05). The following formula was used to calculate the percentage effect of the selected regression characteristics on the dependent variable:
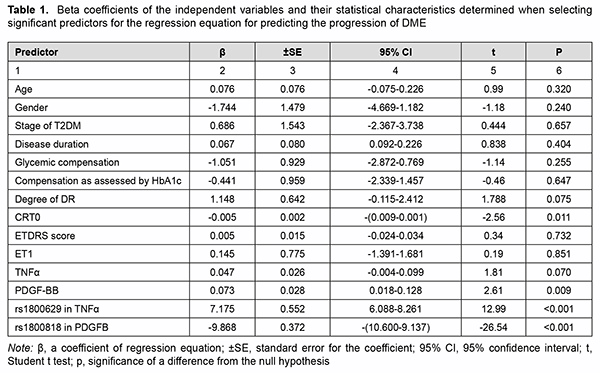
where dk is the percentage effect of the characteristic; βk is beta coefficient of the independent variable of regression equation; and the term of the fraction represents the sum of squares of beta coefficients for all independent variables. Results Table 1 presents beta coefficients of the independent variables and their statistical characteristics determined when selecting significant predictors for the regression equation for predicting the progression of DME.
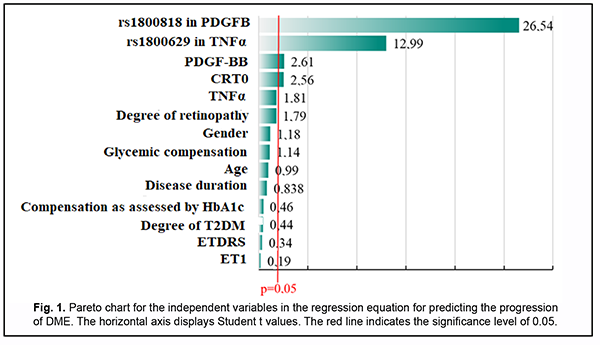
Fig. 1 shows the distribution of variables on the basis of their significance.
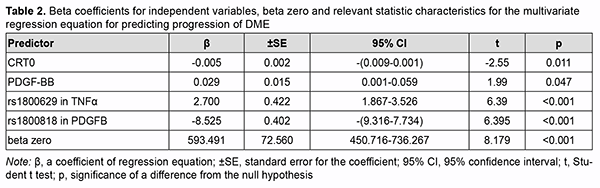
PDGFB rs1800818, TNFα rs1800629, blood PDGF-BB level and baseline central retinal thickness (CRT0) were found to be the most significant variables. A regression model for predicting DME was developed based on these results. Table 2 presents beta coefficients for independent variables, beta zero and relevant statistic characteristics for the multivariate regression equation for predicting progression of DME.
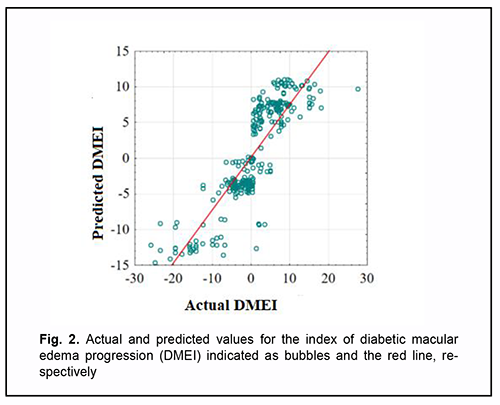
Given the absolute values of beta coefficients, the PDGFB rs1800818 (the predictor with the beta coefficient (|-8.525|) with largest absolute value) was the greatest contributor to the probability of developing recurrent DME, followed by TNFα rs1800629 (|2.700|), PDGF-BB (|0.029|) and CRT0 (|-0.005|). The DMEI was directly related to the dichotomous value of TNFα rs1800629 and blood PDGF-BB, and inversely related to the dichotomous value of PDGFB rs1800818 and CRT0. The percentage contributions of selected independent variables in predicting the dependent variable were found to be as follows: PDGFB rs1800818, 90.88%; TNFα rs1800629, 9.12%; PDGF-BB and CRT0 in combination, 0.001%. The regression equation for predicting the probability of postsurgical DME recurrence in patients with T2DM is as follows: DMEI=593.491-8.525*PDGFB+2.700* TNFα+ +0.029* PDGFBB-0.005* CRT0(3), where DMEI is the index of diabetic macular edema progression; PDGFB is the dichotomous value for the rs1800818 genotype; TNFα is the dichotomous value for the rs1800629 genotype; PDGFBB is the blood PDGF-BB level expressed in ng/mL; and CRT0 is the baseline CRT value expressed in µm. A scatter plot (Fig. 2) shows actual versus predicted DMEI for patients with DMP.
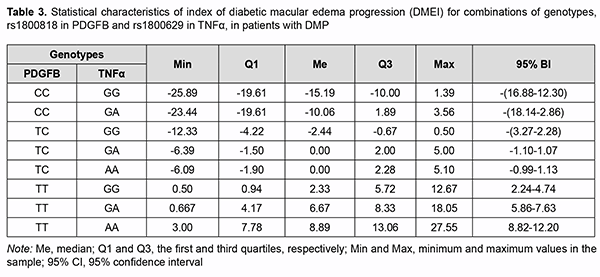
The regression equation was satisfactory, because the coefficient of multiple correlation R (reflects the influence of predictors on the dependent variable) was 0.860, and the coefficient of determination R2 (shows how well a re-gression model fits the data) was 0.704. The statistical significance of the model was satisfactory (F = 219.06; p < 0.001). Table 3 shows DMEI values calculated using formula (3). The genotype combinations are ranked in the order of increasing DMEI values. Possible combinations of boundary values of blood PDGF-BB level and CRT0 were used as blood PDGF-BB and CRT0 characteristics in calculations (Table 4).
A change from negative to positive values was seen in a combination of genotypes, ТС-GA and TC-AА. On the basis of the determined distribution of combinations of PDGFB rs1800818 and TNFα rs1800629 with re-gard to ranked DMEI values calculated using the developed regression equation, we performed the statistical analy-sis of actual DMEI values for patients with DMP on the basis of these genotypes. We differentiated between the following types of the postoperative course of DME on the basis of DMEI val-ues: a regressive postoperative course (DMEI < -1.10) in the presence of genotypes СС-GG, CC-GA and TC-GG; a stable postoperative course (-1.10 ≤ DMEI < 1.13) in the presence of genotypes TC-GA and TC-AA; and a progressive postoperative course (DMEI ≥ 1.13) in the presence of genotypes TT-GG; TT-GA and TT-AA. Discussion This study aimed to solve a challenge facing ophthalmology in general and vitreoretinal surgery in particular to-day, to improve the efficacy of surgical treatment of DMP in patients with T2DM, on the basis of studies of etiolog-ic and pathogenetic components of DMP, and identification of risk factors and prediction of the development and progression as well as post-surgical recurrence. In the first phase of the study, we analyzed the features of DMP in patients with DR and T2DM, and compared various types of current vitreoretinal procedures with regard to efficacy [7, 8]. No significant difference in the inci-dence of DMP and DME was found between patients with NPDR and those with PDR, and the incidence of DMP but not the incidence of DME depended on the duration of T2DM. In the current literature, the concepts of DMP and DME are frequently believed to be identical. In this regard, we agree with Balashevich and Izmailov [1] that DME is a narrower concept than, and one of the most severe mani-festations of DMP. It is for this reason that we have considered the development of DME separately. In addition, in all cases with DME, it was accompanied by certain signs of DMP, whereas there was no macular edema in some cases with DMP. In the presence of proliferative DR, macular edema developed practically in all cases of post-surgical DMP recurrence. Of the cases with DMP recurrence, approximately one third (37.5%) had DME at one month after surgery, and the absolute majority, during further surveillance (80.7% at 3 months, 84.4% at 6 months, and 88.5% at 12 months). This tendency was statistically significant (р < 0.001). There was no difference in the incidence of DME between the methods of treatment (р = 0.129). The key pathogenetic mechanisms of DME in DMP are (a) increased permeability of the capillaries of the blood-retinal barrier (BRB) for plasma proteins resulting in an increased interstitial oncotic pressure (the oncotic mechanism) and (b) arterial hypertension which provokes an increased capillary hydrostatic pressure (the hydro-static mechanism) [1, 14]. The search of risk factors and prognostic factors of DMP in patients with T2DM is still challenging [15]. In addi-tion to clinical factors (disease duration and severity, degree of carbohydrate metabolism compensation, switching over to insulin therapy, smoking, and arterial hypertension) other factors of importance are regulatory factors [9, 15, 16]. It is no doubt that proinflammatory cytokines, such as TNFα, are important for insulin resistance development and mechanisms of T2DM development [17]. It has been demonstrated that TNFα modulates the effect of insulin on receptors by inducing phosphorylation of insulin receptor (IRS1) at serine residues 636/639 and impeding tyro-sine phosphorylation of IRS-1 [18]. This prevents further activation of the PI3K/Akt and ERK/MAP-Kinase Path-ways and glucose consumption [19]. Alternative serine/ tyrosine phosphorylation of IRS1 normally regulates the efficacy of insulin signal transfer, whereas TNFα was shown to stimulate multi-site S/T phosphorylation of IRS1 and IRS2, blocking their interaction with an IR juxtamembrane domain peptide and causing insulin resistance [20]. In our point of view, it is the pathogenetic factors important for the development of DMP that will play a key role in the postoperative development of disease complications and recurrence. In our studies, blood TNFα levels in patients were found to increased correspondingly with the severity of retinopathy (1.2-3.4 times; р < 0.01) [21], and the highest blood TNFα levels were found in PDR. Vitreous cytokine levels in patients with T2DM who underwent posterior vitrectomy were significantly increased, especially in those with PDR [22, 23]. It is under conditions of PDR that Pyrin domain 3 (NLRP3) inflammasomes, the components of pro-inflammatory signalling complexes [22], mostly become activated. After surgery, the activi-ty of the components of this complex increases, which determined the pathogenetic role of proinflammatory cyto-kines and their association with recurrent DMP. In addition to angiogenic cytokines, the study by Raczyńska et al [24] found pro-inflammatory cytokines to be important in the development of retinal ischemia under conditions of diabetes mellitus. A study on the relationship of presurgical blood TNFα and the presence or absence of recurrent DMP found that, in the presence of recurrent FMP, blood TNFα was significantly increased, especially in patients with mild NPDR [21]. Genetic factors, particularly, polymorphisms, can be attributed to the factors contributing to the development of T2DM and its complications [25-29]. A meta-analysis was applied to integrate the findings from 10 studies (with a total of 1425 T2DM patients and 1116 healthy control subjects) to provide an overall assessment whether TNF-α -308G>A (rs1800629) polymorphism is associated with T2DM risk in a population of Han Chinese subjects. The pooled ORs (95% CIs) for TNF-α -308G>A of A vs. G allele and GA+AA vs. GG genotype were 1.63 (1.17–2.25) and 1.47 (1.17–1.85), respectively [29]. A Brazilian study [27] involving 745 outpatients with T2DM, including 331 subjects without DR, 246 with NPDR, and 168 with PDR, demonstrated that the A allele of the –308G>A polymor-phism was (a) more frequent in subjects with PDR than in those with no DR (18.1% vs. 11.5%, corrected P = 0.035), and (b) independently associated with an increased risk of PDR, under a dominant model (adjusted odds ratio [aOR], 1.82; 95% confidence interval [CI], 1.11–2.98). Indian studies [28, 29] showed the association of TNF-α -308G/A (rs1800629) with the development of T2DM and its complications, which was accompanied by increased blood levels of TNFα, IL-6 and SDF-1. A systemic overview by Luna et al [25] noted contradictory results of prior studies aimed at establishing associa-tions between TNF-α -308G/A (rs1800629) and T2DM in various populations. They believe that ethnic differences may play a role in these conflicting results; the results of these studies suggest the need for further investigation. Therefore, the data above pointed to an association of TNF-α -308G/A (rs1800629) with T2DM and its compli-cations (particularly, DR and DMP), and provided evidence on the need for investigating a possible association of this SNP with recurrent DMP after surgical treatment of T2DM patients in a Ukrainian population. We have previously [30] found an association between TNF-α -308G/A (rs1800629) and DMP: those with the minor A/A genotype exhibited a 2.59-fold higher risk (OR = 2.59; 95% CI: 0.89-7.52) of developing DMP. The het-erozygous GA genotype was also found to increase the risk of developing DMP (OR = 1.61; 95% CI: 0. 98-2.65). Our multiplicative-inheritance model analysis found that the minor A allele was associated with a 1.87-fold in-creased risk (OR=1.87; 95% CI: 1.25-2.81) of DMP. We found no significant differences in genotype or allele fre-quencies between patients operated with different techniques, which provided evidence of the genetic homogeneity of the cohort of patients before surgery (p > 0.05). However, previous studies by others [17, 26, 27] found that frequencies of minor AA genotype and A allele were significantly higher in patients with PDR. Therefore, our results were somewhat different from those studies. This may be explained by the fact that, all our patients, irrespective of the stage of their diabetic retinopathy, did have a marked pathological process before treatment, likely due to the presence of unfavorable factors, particularly, those of genetic origin. In other words, our study included patients exhibiting a rapid DMP progression and requiring sur-gery, which resulted in forced selection of DR variants with a very poor clinical course. The model analysis demonstrated that the TNF-α (rs1800629) SNP exerted a significant effect on recurrent DMP at all follow-up time points [30]. Frequencies of risk variants of the TNF-α (rs1800629) SNP were significantly (p < 0.001) higher in patients with recurrent DMP than in those without it. Therefore, our findings directly demon-strated an important role of the TNF-α (rs1800629) SNP in recurrent DMP after surgery, irrespective of DR severity, surgical technique utilized, and follow-up time points. In addition, blood TNFα levels in carriers of risk A/A geno-type and A allele were higher than in carriers of other genotypes; differences in these levels for patients with differ-ent genotypes and for those with different alleles of rs1800629 were significant (p = 0.004 and р=0.002, respective-ly) [11, 30]. It may be noted in the context of discussion on our findings that a meta-analysis [31] reviewed a number of studies on a potential association between SNPs of proinflammatory cytokine genes (TNFα, IL-6 and IL-1β) with T2DM and its complications. Individual SNP analysis showed highest association of TNF-α rs1800629-AA geno-type (OR = 2.75, 95% CI = 1.64-4.59, P = 0.001) with T2DM and diabetic nephropathy [32]. Those authors related it to the fact that TNF-α expression was increased by more than four-folds (n-fold=4.43±1.11), which was accom-panied by a substantial increase in TNF-α blood level in T2DM patients carrying the minor A/A genotype [32]. An-other study [33] found that the minor A/A genotype and minor A allele of rs1800629 significantly increased TNF-α blood level, which was more expressed in patients with complications of T2DM. Therefore, one can identify the following pattern of pathogenetic relationship: minor A allele carriage has a pathogenetic effect on the development of T2DM and its complications which exerts its action due to TNF-α over-expression leading to excessive TNF-α production. We have previously demonstrated the role of increased platelet aggregation in DMP [34]. We continued the re-search in this area, and studied an important regulatory polypeptide, PDGF, which is a transmembrane glycoprotein with mitogenic properties [35], as well as PDGFB rs1800818. PDGF has three isoforms, PDGF-AA, PDGF-AB, and PDGF-BB. The major source of PDGF in blood is from platelet alpha-granules, whereas the source in tissues is from fibroblasts, smooth muscle cells and astrocytes [35, 36]. PDGF-BB is secreted by activated macrophages and nonangiogenic endothelial cells; in contrast to other isoforms of PDGF, it is not rapidly released from the cell, but remains associated with the cell [37]. PDGF-BB is an activator of chemotaxis, cell proliferation, and the expression of the genes that regulate cell proliferation. PDGF-ВВ has an important role under conditions of hypoxia and ischemia, when it contributes to endothelial cell proliferation and neoangiogenesis and improves capillary permeability [36, 37]. Retina-specific expression of PDGF-B in trans-genic mice has been reported to result in severe neovascularization and retinal detachment, which is typical for is-chemic retinopathy [38]. A blood PDGF level in patients with T2DM was found to increase with an increase in severity of DR; particular-ly, blood PDGF levels in patients with NPDR and PDR were increased 1.6-fold to 2.2-fold (p < 0.001) [39]. In addi-tion, blood PDGF level was the same for different surgical techniques. In each group, in the presence of recurrence, blood PDGF levels were 1.3-1.4-fold and statistically significantly (significance by the Mann-Whitney U test, p < 0.001) higher than in the absence of recurrence. The levels of all PDGF isoforms in vitreous were significantly increased in the PDR group, as compared to con-trols, and PDGF-AA and PDGF-BB correlated significantly to the severity of PDR. It has been demonstrated that, under conditions of chronic hyperglycemia, post-receptor resistance to PDGF can develop through activation of protein kinase C (PKC)-delta, MAP kinase and protein tyrosine phosphatase-1 (SHP-1), leading to PDGF receptor-β (PDGFRβ) dephosphorylation, which also increases apoptosis of vascular pericytes. PDGF-B/ PDGFRβ signalling is critical in formation and maturation of BRB through active recruitment of pericytes onto growing retinal vessels. In addition, impaired pericyte recruitment to the vessels showed multiple vascular hallmarks of DR due to BRB disrup-tion [41]. We have previously built a regression model for predicting recurrent DMP based on the baseline PDGF blood level. The model performance measures were satisfactory, and the pre-surgery cut-off PDGF blood level for which the development of recurrent DMP became probable was > 51.8 ng/mL with 89.8% accuracy (p < 0.001), which demonstrated the pathogenetic importance of PDGF in the development of DMP and post-treatment DMP recur-rence [11]. This is the reason why we directed our attention to the PDGFB SNP (rs1800818; locus, 22q13.1; loca-tion, intron of 5′-UTR Variant). The rs1800818 G allele was associated with decreased serum PDGF-BB levels in patients with severe fever with thrombocytopenia syndrome (SFTS) (р = 0.015), and the relative mRNA levels of the at-risk G allele of rs1800818 were lower than those of the A allele in heterozygous cell from acute phase of SFTS patients [42]. In addition, PDGFB rs1800818 A>G was associated with 3-year overall survival rates (A/A gen-otype, 78%; A/G genotype, 69%; [HR 1.37]; G/G genotype, 53%; [HR 2.12]; P = .048) in patients with colorectal liver metastasis who underwent liver resection and receive perioperative bevacizumab-based chemotherapy [43]. It was suggested that variations in genes involved in the angiopoietin and pericyte pathways may be predictive and/or prognostic biomarkers in patients with resected colorectal liver metastasis who receive perioperative bevacizumab-based chemotherapy [43]. We have previously found that the distribution of rs1800818 genotypes under the general model of inheritance showed no association with DMP (χ2=5.38; p=0.068) [44]. The allele frequency comparison under the multiplica-tive model of inheritance, however, did show an association with DMP (χ2 = 5.43; p = 0.020), with those with the minor C allele having a 1.5-lower risk (OR = 0.67; 95% CI: 0.48-0.94) of developing DMP. The findings demon-strated an association between SNP rs1800818 in PDGFB and reduced risk of DMP and post-surgical DMP recur-rence. With regard to a difference in blood PDGFB level among carriers of different genotypes, a significant differ-ence was found only between carriers of different alleles, and blood PDGFB level in carriers of the C allele 1.2-fold and significantly (р=0.001) lower than in carriers of the T allele [44]. Because a protective role of this SNP against DMP and DMP recurrence has been established, it may be sup-posed that it is due to a low blood PDGFB level that carriers of the C allele have a low recurrence risk. Conclusion First, SNP rs1800818 in PDGFB, SNP rs1800629 in TNFα, blood PDGF-BB level, and CRT0 were found to be significant predictors of the risk for DME recurrence. Second, the calculation of DMEI values allowed to divide pos-sible genotype combinations into three groups: those promoting the regression (СС-GG; CC-GA; TC-GG), those with a stable DMEI value (TC-GA and TC-AA), and those promoting the progression of DME.
References 1.Balashevich LI, Izmailov AS. [Diabetic ophthalmopathy]. St. Petersburg: Chelovek; 2012. Russian. 2.Hu XY, Liu H, Wang LN, Ding YZ, Luan J. Efficacy and safety of vitrectomy with internal limiting membrane peeling for diabetic macular edema: a Meta-analysis. Int J Ophthalmol. 2018 Nov 18;11(11):1848-55. 3.Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. Pars plana vitrectomy for diabetic macular edema: A System-atic Review, Meta-Analysis, and Synthesis of Safety Literature. Retina. 2017 May;37(5):886-95. 4.Rinaldi M, dell'Omo R, Morescalchi F, Semeraro F, Gambicorti E, Cacciatore F, et al. ILM peeling in nontractional diabetic macular edema: review and metanalysis. Int Ophthalmol. 2018 Dec;38(6):2709-14. 5.Doi N, Sakamoto T, Sonoda Y, Yasuda M, Yonemoto K, Arimura N, et al. Comparative study of vitrectomy versus intravitreous triamcinolone for diabetic macular edema on randomized paired-eyes. Graefes Arch Clin Exp Ophthalmol. 2012 Jan;250(1):71-8. doi: 10.1007/s00417-011-1777-7. 6.Xiao K, Dong YC, Xiao XG, Liang SZ, Wang J, Qian C, Wan GM. Effect of pars plana vitrectomy with or without cataract surgery in patients with diabetes: a systematic review and meta-analysis. Diabetes Ther. 2019 Oct;10(5):1859-68. doi: 10.1007/s13300-019-0672-9. 7.Panchenko Yu. Effectiveness of different methods of surgical treatment of diabetic maculopathy in patients with type 2 diabetes. American Science Journal. 2019;28(2):27-34. 8.Panchenko Yu. Possibilities and effectiveness of cataract phacoemulsification, closed subtotal vitrectomy and panretinal laser coagu-lation in diabetic maculopathy treatment in patients with type 2 diabetes. East European Science Journal. 2019;7(47 part 2):50-6. 9.Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology. 2017;24(4):229-41. 10.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Dia-bet Stud. 2015 Spring-Summer;12(1-2):159-95. 11.Mogilevskyy SIu, Panchenko IuO, Ziablytsev SV. New risk factors for post-surgical recurrent diabetic maculopathy in type 2 dia-betus mellitus. J Ophthalmol (Ukraine). 2019;5(490):9-17. 12.Kamoi K, Takeda K, Hashimoto K, Tanaka R, Okuyama S. Identifying risk factors for clinically significant diabetic macula edema in patients with type 2 diabetes mellitus. Curr Diabetes Rev. 2013 May;9(3):209-17. 13.Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Practice. 2013 Jun;100(3):298-305. 14.Cunha-Vaz J. The blood-retinal barrier in the management of retinal disease: EURETINA Award Lecture. Ophthalmologica. 2017; 237 (1):1-10. 15.Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Practice. 2013 Jun; 100 (3):298-305. 16.Kamoi K. Identifying risk factors for clinically significant diabetic macula edema in patients with type 2 diabetes mellitus. Curr Dia-betes Rev. 2013; 9 (3):209-17. 17.Liu C, Feng X, Li Q, Wang Y, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes. A systematic review and meta-analysis. Cytokine. 2016 Oct; 86:100-9. 18.IRS1 – Insulin receptor substrate 1 – Homo sapiens (Human) – IRS1 gene & protein". Available on: www.uniprot.org. Retrieved 2016-04-21. 19.Takaguri A. Elucidation of a new mechanism of onset of insulin resistance: effects of statins and tumor necrosis factor-α on insulin signal transduction. Yakugaku Zasshi. 2018; 138 (11):1329-34. 20.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012; 55 (10):2565-82. 21.Panchenko YuO. [Tumor necrosis factor-alpha (TNFα) and recurrence of diabetic maculopathy after surgery in patients with type 2 diabetes]. Ophthalmology. 2019; 2:65-76. Ukrainian. 22.Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017 Dec; 95 (8):803-8. 23.Loukovaara S, Sandholm J, Aalto K, Liukkonen J, Jalkanen S, Yegutkin GG. Deregulation of ocular nucleotide homeostasis in patients with diabetic retinopathy. J Mol Med (Berl). 2017; 95 (2):193-204. 24.Raczyńska D, Lisowska KA, Pietruczuk K, Borucka J, Ślizień M, Raczyńska K, et al. The Level of Cytokines in the Vitreous Body of Severe Proliferative Diabetic Retinopathy Patients Undergoing Posterior Vitrectomy. Curr Pharm Des. 2018; 24 (27):3276-81. 25.Luna GI, da Silva IC, Sanchez MN. Association between -308G/A TNFA Polymorphism and Susceptibility to Type 2 Diabetes Mellitus: A Systematic Review. J Diabetes Res. 2016; 2016:6309484. 26.Liu ZH, Ding YL, Xiu LC, Pan HY, Liang Y, Zhong SQ, et al. A meta-analysis of the association between TNF-α -308G>A pol-ymorphism and type 2 diabetes mellitus in Han Chinese population. PLoS One. 2013; 8 (3):e59421. 27.Sesti LF, Crispim D, Canani LH, Polina ER, Rheinheimer J, Carvalho PS, Gross JL, Santos KG. The -308G>A polymorphism of the TNF gene is associated with proliferative diabetic retinopathy in Caucasian Brazilians with type 2 diabetes. Invest Ophthal-mol Vis Sci. 2015 Jan 29; 56 (2):1184-90. 28.Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-α and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015 Jul 1; 565 (1):62-7. 29.Doody NE, Dowejko MM, Akam EC, Cox NJ, Bhatti JS, Singh P, et al. The role of TLR4, TNF-α and IL-1β in type 2 diabetes mellitus development within a North Indian population. Ann Hum Genet. 2017 Jul; 81 (4):141-6. 30.Panchenko IuO. Value of TNF-α (rs1800629) polymorphism in recurrent maculopathy after surgery in a Ukrainian population of T2DM patients. J Ophthalmol (Ukraine).2019;6:15-22. 31.Zhao Y, Li Z, Zhang L, Zhang Y, Yang Y, Tang Y, Fu P. The TNF-alpha -308G/A polymorphism is associated with type 2 diabe-tes mellitus: an updated meta-analysis. Mol Biol Rep. 2014 Jan; 41 (1):73-83. 32.Hameed I, Masoodi SR, Malik PA, Mir SA, Ghazanfar K, Ganai BA. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018 Jun 30; 661:51-59. doi: 10.1016/j.gene.2018.03.095. 33.Umapathy D, Krishnamoorthy E, Mariappanadar V, Viswanathan V, Ramkumar KM. Increased levels of circulating (TNF-α) is associated with (-308G/A) promoter polymorphism of TNF-α gene in Diabetic Nephropathy. Int J Biol Macromol. 2018 Feb; 107 (B):2113-21. 34.37. Mogilevskyy SIu, Panchenko YuO, Ziablitsev SV, Ziablitsev DS. Influence of local and systemic factors of type 2 diabetes mellitus on the functional status of platelets in patients with diabetic retinopathy and maculopathy. J Ophthalmol (Ukraine). 2018;6:23-9. 35.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009 Jan; 23 (1):153-63. 36.Rodriguez A, Friman T, Kowanetz M, van Wieringen T, Gustafsson R, Sundberg C. Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-β1 in normal skin in vivo. Am J Pathol. 2013 Jun; 182 (6):2132-46. 37.Hata N, Shinojima N, Gumin J, Yong R, Marini F, Andreeff M, Lang FF. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery. 2010 Jan; 66 (1):144-56; discussion 156 7. 38.Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, et al. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002; 43:2001-6. 39.Panchenko YuO, Mogilevskyy SIu, Ziablitsev SV. [The effect of platelet-derived growth factor (PDGF) on the development of relapses in the surgical treatment of diabetic maculopathy in patients with type 2 diabetes]. Archive of Ophthalmology of Ukraine. 2019;7(3):18-23. Ukrainian. 40.Praidou A, Klangas I, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, Dimitrakos S. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009 Feb; 34 (2):152-61. 41.Park HY, Kim IT, Park CK. Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coher-ence tomography. Br J Ophthalmol. 2011; 95 (9):1223-8. 42.Zhang XA, Guo CT, Lu QB, Hu JG, Cui N, Yang ZD, et al. The platelet derived growth factor-B polymorphism is associated with risk of severe fever with thrombocytopenia syndrome in Chinese individuals. Oncotarget. 2016 May 31; 7 (22):33340-9. 43.Stremitzer S, Zhang W, Yang D, Ning Y, Stintzing S, Sebio A, et al. Genetic variations in angiopoietin and pericyte pathways and clinical outcome in patients with resected colorectal liver metastases. Cancer. 2015 Jun 1;121(11):1898-905. 44.Panchenko YuO. [Association of rs1800818 PDGFB gene polymorphism with recurrence of diabetic maculopathy after surgery in patients with type 2 diabetes]. Bulletin of problems of biology and medicine. 2019;4;1(153):138-42. [Ukranian] Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|