J.ophthalmol.(Ukraine).2021;4:19-25.
|
http://doi.org/10.31288/oftalmolzh202141925 Received: 24 June 2021; Published on-line: 16 August 2021 Vitreous VEGF levels among patients with proliferative diabetic retinopathy depending on the general clinical status and ocular status Vira S. Ponomarchuk, L. M. Velychko, M. M. Umanets SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine»; Odesa (Ukraine) E-mail: v.zavodnaya@gmail.com TO CITE THIS ARTICLE: Ponomarchuk Vira S, Velychko LM, Umanets MM. Vitreous VEGF levels among patients with proliferative diabetic retinopathy depending on the general clinical status and ocular status. J.ophthalmol.(Ukraine). 2021;4:19-25. http://doi.org/10.31288/oftalmolzh202141925 Background: The most common cause of visual impairment in patients with diabetic retinopathy is the pathology progression to the proliferative stage which is accompanied by apparent fibrovascular proliferation, development of tractional retinal detachment and/or vitreous hemorrhage. Vascular endothelial growth factor (VEGF) is the most important in the pathogenesis of ocular microvascular changes in diabetes. Purpose: To assess vitreous VEGF levels in the proliferative diabetic retinopathy (PDR) patients not initially treated with anti-VEGF agents, depending on the general clinical status and ocular status. Material and Methods: Forty-one patients (45 eyes) aged 19 to 81 years with neovascular glial PDR and epiretinal membrane with a marked proliferative component were involved in the study. Each patient underwent a 25G three-port vitrectomy during which a vitreous specimen was collected. Vitreous VEGF levels were determined by a three-step enzyme-linked immunosorbent assay. Results: We managed to assess total vitreous VEGF levels in 44 of the 45 eyes. Patients with PDR had elevated total vitreous VEGF levels, with a mean value of 757.69 ± 117 pg/ml, confirming the involvement of VEGF in pathological intraocular angiogenesis. There was a significant difference in vitreous VEGF levels between PDR patients with a fibrovascular membrane with a marked proliferative component (997.0±151.8 pg/ml) and those with a fibrovascular membrane with a moderate proliferative component (244.9 ± 53.7 pg/ml) (F = 10.3; р = 0.0025). Keywords: vascular endothelial growth factor, proliferative diabetic retinopathy
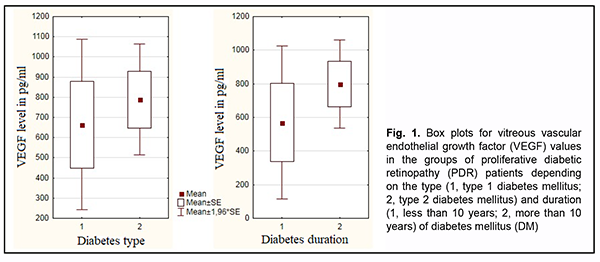
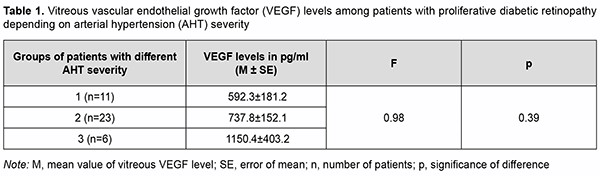
Introduction Ocular complications of diabetes mellitus (DM) are a major cause of blindness and visual impairment among the population aged younger than 50 years. The most common cause of visual impairment in patients with diabetic retinopathy is the pathology progression to the proliferative stage which is accompanied by apparent fibrovascular proliferation, development of tractional retinal detachment and/or vitreous hemorrhage [1-4]. Vascular endothelial growth factor (VEGF) is believed to be the most important in the pathogenesis of ocular microvascular changes in diabetes. In 1982 the name vascular endothelial growth factor was first used to denote endothelium specific mito-genic activity isolated from calf retina [5]. VEGF-A exists as a homodimeric glycoprotein comprised of two identical 23 kDa subunits, and is the most important for control of endothelial cell formation and proliferation during human angiogenesis [6]. VEGF-A expression is activated under conditions of hypoxia either through an increased VEGF-A transcription or through a 3- to 8-increase in the VEGF-A mRNA half time [7, 8]. Low constitutive expression of VEGF mRNA has been demonstrated in almost all tissues of the normal eye, most notably in the ciliary body, con-junctiva, RPE/choroid, and lens [5]. Pericytes, vascular endothelial cells, retinal glia and Muller cells are involved in the synthesis of this cytokine in the retina [9, 10]. The receptors for VEGF-A have been found in endothelial cells and pericytes of retinal and choroidal vessels, retinal glia cells, retinal pigment epithelial cells, and corneal endothelial cells [11]. There is much experimental evi-dence of its proangiogenic role and its effect on retinal vascular permeability. VEGF-A is mitogenic for cultured vascular and lymphatic endothelial cells and stimulates their migration and tube formation, the so called ‘angiogenesis in vitro’ [5, 12]. The above properties contribute to its significant role in the pathogenesis of diabetic retinopathy. The involvement of VEGF-A in retinal lesions in diabetes has been demonstrated in experimental animal models [13, 14]. VEGF levels in the vitreous, subretinal fluid and serum were found to vary with pathological changes in the reti-na and vitreous [15-17]. In addition, it is in proliferative diabetic retinopathy (PDR) that vitreous VEGF levels are commonly highest. There are numerous studies on VEGF in PDR, but there is no general agreement on the correla-tion of VEGF levels with the general clinical status and ocular status of patients with DM. Therefore, the purpose of the study was to examine the relationships of the general clinical status (type and duration of diabetes and severity of arterial hypertension) and ocular status with total VEGF vitreous levels among PDR patients not initially treated with anti-VEGF agents. Material and Methods This was a pilot open prospective interventional study of 41 patients (45 eyes) aged 19 to 81 years with a neo-vascular glial form of proliferative diabetic retinopathy (PDR) as graded by the Pasyechnikova-Naumenko system [18]. There were 28 women (32 eyes) and 13 men (13 eyes). Of the 41 patients, 9 (11 eyes; age, 19 to 61 years) had type 1 diabetes and 32 (34 eyes; age, 19 to 61 years) had type 2 diabetes. The blood glucose levels ranged from 7.0 mmol/l to 14 mmol/l, and diabetes duration ranged from 2 years to 20 years. The proliferative process was characterized by the development of fibrovascular epiretinal membrane (FERM) in all patients. In addition, no retinal detachment was seen in two eyes, a threat to the macula from tractional reti-nal detachment (TRD), in 14 eyes, TRD with macular involvement, in 22 eyes, and combined tractional and rheg-matogenous retinal detachment (TRRD), in 7 eyes. Moreover, vitreous hemorrhage was observed in 37 eyes (par-ticularly, retrohyaloid hemorrhage, in 3 eyes, and partial vitreous hemorrhage, in 34 eyes). Patients were divided into three groups depending on the blood pressure (BP) graded according to the 2007 WHO grading system in order to determine the relationship between the severity of arterial hypertension and vitre-ous VEGF levels in patients with PDR. Group 1 comprised 11 patients (11 eyes) with BP ranging from 140 mmHg to 160 mmHg, Group 2, 23 patients (27 eyes) with BP ranging from 160 mmHg to 180 mmHg, and Group 3, 6 pa-tients (6 eyes) with BP above 180 mmHg. Preoperative visual acuity ranged from light perception with accurate projection to 0.2, and was worse than 0.1 in 70.5% of eyes. Baseline intraocular pressure (IOP) was within the normal range, and ranged from 17.0 mm Hg to 23.0 mmHg. Initial complicated cataract was observed in 15 eyes and 30 eyes had an intraocular lens (IOL) im-planted. In addition, 34 eyes had a recent history of panretinal laser photocoagulation. Each patient underwent an eye examination which included visual acuity assessment, refractometry, tonome-try, static automated perimetry, biomicroscopy, gonioscopy and ophthalmoscopy. Patients with a history of vitrec-tomy, prior anti-VEGF injections, or the presence of uveitis (or intraocular inflammation), iris rubeosis, elevated IOP, total vitreous hemorrhage, central retinal vein or branch occlusion, or central retinal artery occlusion were ex-cluded. Each patient underwent a 25G three-port vitrectomy using an Alcon Constellation 25-G vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA) and OMS-800 OFFISS microscope (Topcon, Tokyo, Japan). Pre-operative informed consent was taken from all the patients involved in the study. Forty-five vitreous specimens were used in immunological studies. A 0.2-ml specimen from the anterior vitreous was collected into a disposable tube connected to the aspiration line of the vitrectomy probe. The specimens obtained were frozen and maintained at -20о С until immunological assays were performed. Total VEGF levels were determined by a three-step enzyme-linked immunosorbent assay (ELISA). A test system and VEGF ELISA kits (Vector Best, Novosibirsk, Russia; ISO 13485) were employed as per the manufacturer’s instruction. Photometric measurements were performed at 450 nm on an ELISA plate reader (Stat Fax 2100, Awareness Technologies Inc, Palm City, FL). Statistica 9 (StatSoft, Tulsa, OK, USA) software was used for statistical analysis. Student t test was used to compare independent samples. Univariate analysis of variance was used to examine differences among the three groups. The level of significance p < 0.05 was assumed. In addition, the Mann-Whitney test was used for detecting significant differences between two groups, whereas the Kruskal-Wallis test was used for detecting significant differ-ences among the three groups. The study protocol conformed to the tenets of the Declaration of Helsinki, and was approved by the Bioethics Committee of the Filatov Institute (Protocol No. 1 dated October 15, 2018). Written informed consent was obtained from all patients. Results We managed to assess total vitreous VEGF levels in 44 of the 45 eyes with a neovascular glial form of PDR (range, 15.6 pg/ml to 2659 pg/ml; mean value, 757.69 ± 117 pg/ml). Total vitreous VEGF level was unable to be measured in one eye due to hemolysis. Mean total vitreous VEGF level was 664.7 ± 238.4 pg/ml for patients with T1DM and 788.7±137.6 pg/ml for patients with T2DM. Patients with a neovascular glial form of PDR were divided in two groups depending on the duration of diabetes, group 1 of 9 patients (9 eyes) with a diabetes duration of less than 10 years, and group 2 of 32 patients (36 eyes) with a diabetes duration of more than 10 years. Mean total vitreous VEGF level was 569.4 ± 277.1 pg/ml for the former group and 799.5 ± 130.6 pg/ml for the latter group. The Mann-Whitney test showed no significant difference in vitreous VEGF level between groups with T1DM and T2DM (р=0.62) as well as between groups with a diabetes duration of less than 10 years and more than 10 years (р = 0.39) (Fig. 1).
Therefore, we found that neither the type nor the duration of DM had an effect on vitreous VEGF level in pa-tients with a neovascular glial form of PDR. Table 1 shows mean vitreous VEGF levels for patients with PDR depending on the severity of arterial hyperten-sion. Vitreous VEGF levels did not depend on the severity of arterial hypertension (Table 1; р=0.39).
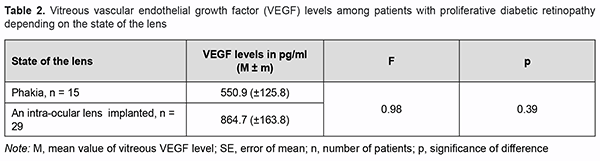
Because others have reported on the risk for the development of secondary neovascular glaucoma after cata-ract phacoemulsification in PDR patients [19, 20], we decided to determine vitreous VEGF levels for patients with PDR depending on the state of the lens (Table 2).
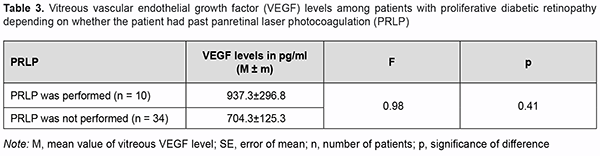
There was no significant difference in vitreous VEGF levels among the groups with different states of the lens (Table 2; р=0.17). We also assessed the effect of past panretinal laser photocoagulation on vitreous VEGF levels in 34 eyes with PDR (Table 3).
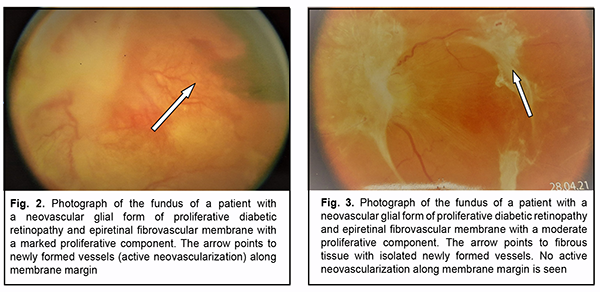
Although no significant effect of panretinal laser photocoagulation on vitreous VEGF levels in patients with PDR was observed (р=0.41), mean vitreous VEGF levels were higher in patients with no history of panretinal laser photocoagulation. On ophthalmoscopy, fibrovascular membranes varied in the degree of proliferation. That is, in 30 eyes, fibro-vascular membranes showed newly formed vessels of various sizes (predominantly small vessels with active neo-vascularization along membrane margin) and were classified as those with a marked proliferative component (Fig. 2). In addition, in 14 eyes, epiretinal membranes showed fibrous tissue with a smaller number of newly formed ves-sels (and no active neovascularization along membrane margin) and were classified as those with a moderate pro-liferative component (Fig. 3).
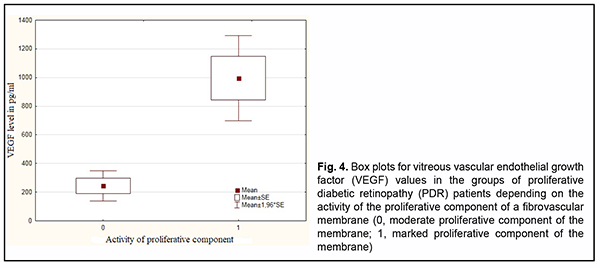
Fig. 4 shows vitreous VEGF levels in patients with PDR depending on the activity of the proliferative component of a fibrovascular membrane. There was a significant difference (р=0.002) in vitreous VEGF levels between PDR patients with a fibrovascular membrane with a marked proliferative component (997.0±151.8 pg/ml) and those with a fibrovascular membrane with a moderate proliferative component (244.9 ± 53.7 pg/ml). Non-parametric Mann-Whitney test confirmed a significant difference in vitreous VEGF levels (p=0.007) between PDR patients with a marked proliferative component and those with a moderate proliferative component (F = 10.3; р = 0.0025).
Discussion It has been reported that VEGF levels in the vitreous, subretinal fluid and serum were clearly correlated with the severity of PDR and that VEGF levels in the vitreous could be significantly elevated in the presence of active intra-ocular neoangiogenesis [21]. Kocak and colleagues [22] found that the levels of proinflammatory cytokines (including VEGF) in the vitreous were higher in diabetic patients than the non-diabetics who underwent pars plana vitrectomy. The current study included 41 patients (45 eyes) with a neovascular glial form of PDR. Vitreous VEGF levels in 44 eyes ranged from 15.6 pg/ml to 2659 pg/ml with a mean value of 757.69 ± 117 pg/ml. Patients with iris rubeosis or total vitreous hemorrhage were excluded from the study because it has been reported that vitreous VEGF levels in such patients could be significantly elevated [23, 24]. We found that vitreous VEGF levels were not significantly effected by the type or duration of diabetes, severity of arterial hypertension, state of the lens or whether the patient had or had not past panretinal laser photocoagula-tion. Our findings are in agreement with those reported by others regarding the effect of the type or duration of dia-betes on vitreous VEGF levels [15]; however, they are in disagreement with those reported by others regarding the effect of the state of the lens and whether the patient had past panretinal laser photocoagulation. Although there have been reports on an increased risk of secondary neovascular glaucoma after phacoemulsification with IOL implantation in patients with PDR [19, 20], we did not find this in our patients. In addition, others have reported that plasma VEGF levels were higher in the PDR patients who did not have past panretinal laser photocoagulation than in those who did [25, 26]. In the current study, vitreous VEGF levels were higher in the PDR patients who did not have past panretinal laser photocoagulation than in those who did, although the difference was not significant. In studies by Neroev and colleagues [15, 27], VEGF levels in the vitreous, subretinal fluid and serum were high-est in the eyes operated for complicated PDR without preliminary anti-VEGF therapy, with mean values of 1151.6 pg/ml, 3490 pg/ml and 226.4 pg/ml for the vitreous, subretinal fluid, and serum, respectively, compared to 205.6±175.0 pg/ml, 775.4±560 pg/ml, and 140.5 ± 64.2 pg/ml, respectively, in the eyes operated for PDR with a stable course without preliminary anti-VEGF therapy. In addition, among eyes with PDR, tear VEGF-A levels were higher in those with a fibrovascular epiretinal membrane compared to those with a fibroglial epiretinal membrane. Moreover, tear VEGF-A levels of 3490 pg/ml or above were significantly associated with the presence of active ne-ovascularization within the eye, and may be used as a prognostic criterion for the presence of active neovasculari-zation within an eye with opaque media [15, 27]. Habibah and colleagues [17] investigated the concentrations of VEGF in vitreous and serum samples, analyzed the ratio, and compared among PDR subgroups. Their study included 17 eyes of patients with PDR, identified as the PDR group which was divided into three subgroups (vitreous hemorrhage [VH], VH with fibrotic tissues, and trac-tional retinal detachment), and five control eyes. The VEGF-A concentrations were calculated by ELISA. The VEGF-A concentrations in vitreous samples were significantly higher in the PDR group (630.72 ± 342.81 pg/mL) compared with those in the control group (153.58 ± 145.85 pg/mL). The VEGF-A concentrations in vitreous sam-ples were highest in the VH group and lowest in the VH with fibrotic tissue subgroup. Habibah and colleagues [17] concluded that high concentrations of VEGF in vitreous samples of PDR eyes indicate its local related activity in PDR pathology. A common feature of all the patients of the current study was the presence of a fibrovascular membrane in the eye. We divided these membranes into two types depending on their proliferating activity: (a) a fibrovascular mem-brane with a marked proliferative component, which showed newly formed vessels of various sizes (predominantly small vessels with active neovascularization along membrane margin) and (b) a fibrovascular membrane with a moderate proliferative component, which showed fibrous tissue with a smaller number of newly formed vessels (and no active neovascularization along membrane margin). There was a significant difference in vitreous VEGF levels between PDR patients with a marked proliferative component and those with a moderate proliferative com-ponent (F = 10.3; р = 0.0025). Therefore, we found that, among patients with a neovascular glial form of PDR, total vitreous VEGF levels had a mean value of 757.69±117 pg/mL, and were almost fourfold and statistically significantly (р = 0.002) higher in those with a fibrovascular membrane with a marked proliferative component compared to those with a fibrovascu-lar membrane with a moderate proliferative component.
References 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. 2.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581-611. doi: 10.1210/er.2003-0027. 3.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for diabetic macular edema. N Engl J Med. 2015 Mar 26;372(13):1193-203. doi: 10.1056/NEJMoa1414264. 4.Agarwal D, Gelman R, Prospero Ponce C, et al. Vitreomacular Interface in Diabetic Retinopathy. J Ophthalmol. 2015;2015:392983. doi: 10.1155/2015/392983.. 5.Schlingemann RO, van Hinsbergh VW. Role of vascular permeability factor / vascular endothelial growth factor in eye disease. Br J Ophthalmol. 1997 Jun;81(6):501-12. doi: 10.1136/bjo.81.6.501. 6.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983-5. doi: 10.1126/science.6823562. 7.Bhisitkul RB. Vascular endothelial growth factor biology: clinical implications for ocular treatments. Br J Ophthalmol. 2006 Dec;90(12):1542-7. doi: 10.1136/bjo.2006.098426. 8.Levy NS, Chung S, Furneaux H, et al. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998 Mar 13;273(11):6417-23. doi: 10.1074/jbc.273.11.6417. 9.Adamis AP, Shima DT, Yeo KT, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):631-8. doi: 10.1006/bbrc.1993.1671. 10.Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995 Dec;113(12):1538-44.. doi: 10.1001/archopht.1995.01100120068012. 11.Ferrara N, Gerber HP, LeCouter JN. The biology of VEGF and its receptors. Nat Med. 2003 Jun;9(6):669-76. doi: 10.1038/nm0603-669. 12.Takahashi H, Shibuya M.. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005 Sep;109(3):227-41. doi: 10.1042/CS20040370. 13.Gilbert RE, Vranes D, Berka JL, et al. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab In-vest. - 1998 Aug;78(8):1017-27. 14.Murata T, Nakagawa K, Khalil A, et al. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996 Apr;74(4):819-25.. 15.Neroev VV, Zaytseva OV, Balatskaya NV, Kurchaeva ZV. [Local and systemic VEGF-A production in complicated proliferated diabetic retinopathy]. Meditsinskaya immunologiya. 2016;18(4):357-64. Russian. doi: 10.15789/1563-0625-2016-4-357-364. 16.Chen HJ, Ma Z-Z, Li Y, et al. Change of Vascular Endothelial Growth Factor Levels following Vitrectomy in Eyes with Prolifera-tive Diabetic Retinopathy. J Ophthalmol. 2019 Oct 23;2019:6764932. doi: 10.1155/2019/6764932. 17.Muhiddin HS, Kamaruddin MI, Ichsan AM, et al. Vitreous and Serum Concentrations of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor in Proliferative Diabetic Retinopathy. Clin Ophthalmol. 2020 Jun 9;14:1547-1552. doi: 10.2147/OPTH.S248812. 18.Pasyechnikova NV. [Laser treatment in fundus diseases]. Kyiv: Naukova dumka; 2007. Russian. 19.Sakamoto M, Hashimoto R, Yoshida I, et al. Risk factors for neovascular glaucoma after vitrectomy in eyes with proliferative dia-betic retinopathy. Clin Ophthalmol. 2018 Nov 14;12:2323-2329. doi: 10.2147/OPTH.S184959. 20.Kadonosono K, Matsumoto S, Uchio E, et al. Iris neovascularization after vitrectomy combined with phacoemulsification and intra-ocular lens implantation for proliferative diabetic retinopathy. Ophthalmic Surg Lasers. Jan-Feb 2001;32(1):19-24. 21.Tojo N, Kashiwagi Y, Nishitsuka K, et al. Interactions between vitreous-derived cells and vascular endothelial cells in vitreoretinal diseases. Acta Ophthalmologica. Acta Ophthalmologica. 88(5):564-70. DOI: 10.1111/j.1755-3768.2008.01466.x. 22.Kocak N, Alacacioglu I, Kaynak S, et al. Comparison of vitreous and plasma levels of vascular endothelial growth factor, interleu-kin-6 and hepatocyte growth factor in diabetic and non-diabetic retinal detachment cases. Ann Ophthalmol (Skokie). 2010;42 Spec No:10-4. 23.Kabesha TB, Glacet-Bernard A, Rostaqui O, et al. [Anti-VEGF therapy in the treatment of anterior segment neovascularization sec-ondary to central retinal vein occlusion]. J Fr Ophtalmol. 2015 May;38(5):414-20. doi: 10.1016/j.jfo.2014.11.007. 24.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopa-thy. Am J Ophthalmol. 1994 Oct 15;118(4):445-50. doi: 10.1016/s0002-9394(14)75794-0. 25.Lip PL, Belgore F, Blann AD, et al. Plasma VEGF and soluble VEGF receptor FLT-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatment. Invest Ophthalmol Vis Sci. 2000 Jul;41(8):2115-9. 26.Mohamed TA, Mohamed Sel-D. Effect of pan-retinal laser photocoagulation on plasma VEGF, endothelin-1 and nitric oxide in PDR. Int J Ophthalmol. 2010;3(1):19-22. doi: 10.3980/j.issn.2222-3959.2010.01.05. 27.Neroev VV, Slepova OS, Zaitseva OV. [Features of local and systemic endothelin production in complicated proliferated diabetic retinopathy]. Rossiiskii oftalmologicheskii zhurnal. 2015;8(3):31-7. Russian. Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|