J.ophthalmol.(Ukraine).2021;5:77-83.
|
http://doi.org/10.31288/oftalmolzh202157783 Received: 04 March 2021; Published on-line: 23 October 2021 Avoiding errors in the diagnosis of polypoidal choroidal vasculopathy in patients with age-related macular degeneration (Part 2: Example cases) N. S. Lutsenko, O. A. Rudycheva, O. A. Isakova, T. S. Kyrylova Zaporizhzhia Medical Academy of Post-Graduate Education; Zaporizhzhia (Ukraine) E-mail: rudychevaolga5@gmail.com TO CITE THIS ARTICLE: Lutsenko NS, Rudycheva OA, Isakova OA, Kyrylova TS. Avoiding errors in the diagnosis of polypoidal choroidal vasculopathy in patients with age-related macular degeneration (Part 2: Example cases. J.ophthalmol.(Ukraine).2021;5:77-83. http://doi.org/10.31288/oftalmolzh202157783
Polypoidal choroidal vasculopathy (PCV) is difficult to diagnose subtype of neovascular age-related macular degeneration (nAMD). Although advances in optical coherence tomography (OCT) and OCT angiography (OCTA) have allowed much improved diagnosis of PCV when indocyanine green angiography (ICGA), a gold-standard for diagnosing PCV, is unavailable, the task may still represent a challenge even for the most experienced ophthalmologists. Numerous diagnostic errors (including failure in elicitation or interpretation of symptoms or signs) may be partially due to the absence of a universal algorithm for identification of the pathology. We believe that highlighting the pathological features of importance to confirm diagnosis, and developing a clearly defined sequence of steps for interpretation of OCT and OCTS results will be a valuable approach to improved diagnosis of this type of nAMD. Three nAMD cases that had OCT signs consistent with PCV and required further differential diagnostic assessment to confirm or exclude PCV are presented as examples demonstrating the efficacy of the proposed algorithm. In these example cases, the OCT findings suggestive of PCV are reported, and OCTA segmentation steps with the related actions as per the proposed algorithm are described. The example cases demonstrate that the stepwise assessment of OCT and OCTA results for patients with nAMD allows to determine reliably whether PCV is present or not (i.e., whether the diagnosis should be confirmed or refuted). Key words: optical coherence tomography, optical coherence tomography angiography, age-related macular degeneration, polypoidal choroidal vasculopathy, differential diagnosis
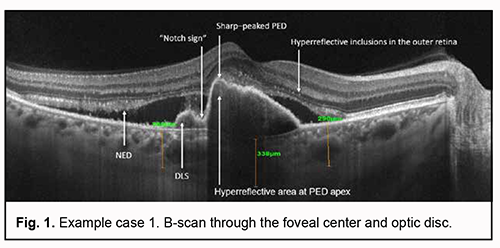
Although advances in diagnostic technologies for eye diseases and advent of optical coherence tomography (OCT) and OCT angiography (OCTA) have allowed much improved diagnosis of various fundus pathologies [1-4], diagnostic errors (including failure in elicitation or interpretation of symptoms or signs) and cases of misdiagnosis still occur [5, 6]. Age-related macular degeneration (AMD) is no exception. Diagnosis of various forms of AMD and various types of neovascularization may still represent a challenge even for the most experienced ophthalmologists. Polypoidal choroidal vasculopathy (PCV) is difficult to diagnose subtype of neovascular AMD [7]. OCT as well as OCTA is an important method for the diagnosis of PCV as a subtype of neovascular AMD [8, 9]. The development of a step-by-step algorithm of the OCT and OCTA diagnosis of PCV, along with its subsequent incorporation in clinical practice, is an approach that can help doctors improve the accurateness of diagnosis and interpret OCT data more effectively [9, 10, 11]. The purpose of this paper was to demonstrate the clinical use of the algorithm for OCTA-based differential diagnosis of PCV in patients with supposed PCV. Materials and Methods Three example cases are presented. These cases include selected patients with neovascular forms of AMD who exhibited OCT features that are characteristic of PCV. Patients underwent an eye examination including visual acuity, perimetry and tonometry. The multimodal fundus imaging included ophthalmoscopy, scanning laser ophthalmoscopy, OCT, en face OCT and OCTA. AMD diagnosis was based on the 2018 NICE guidelines [12], including recommendations on classifying AMD. This study was performed within the framework of a planned research design for the department project (VN.R.01.05-19 №0019U101932). The study protocol was approved by the Bioethics Committee (Committee Minutes dated 14.01.2019). Informed consent was obtained from all participants. The AngioVue OCTA system (RTVue XR OCT Avanti, Optovue, Inc., Fremont, CA) was used for split-spectrum amplitude-decorrelation angiography (SSADA) measurements with OCT and OCTA. Retinal OCT was performed using Cross Line, Retina Map, and 3D Widefield scans. We used OCT to measure choroidal thickness manually at the foveal center and determine maximum thickness. The AngioRetina 3 × 3 mm and 6 × 6 mm scan protocols were used to perform OCTA in the macular area. Angiography was assessed using manual and automatic segmentation modes. The algorithm we had proposed and reported [13] was used for analysis of OCTA results. Example case 1 In this case, OCT showed a high retinal pigment epithelial detachment (PED) with a sharp peak characteristic of PCV, as well as the following characteristic OCT features strongly suggestive of PCV: a “notch sign”, hyperreflective area adjacent to the RPE, a double-layer sign (DLS), and additional OCT features indicating the activity of a suspected neovascular membrane (neuroepithelial detachment (NED) and hyperreflective inclusions in the outer retina). Of note was the area of increased reflectivity above PED, at the fovea, which was consistent with diffuse edema of photoreceptors, but it was difficult to exclude the presence of neovascularization in this zone based on the results of OCT only. Therefore, in this example case, it was impossible to reliably exclude the presence of mixed choroidal neovascularization based on OCT only. The choroidal thickness at the fovea was 338 mm, which was above normal values. OCTA-based analysis was used to confirm or reject the diagnosis of PCV.
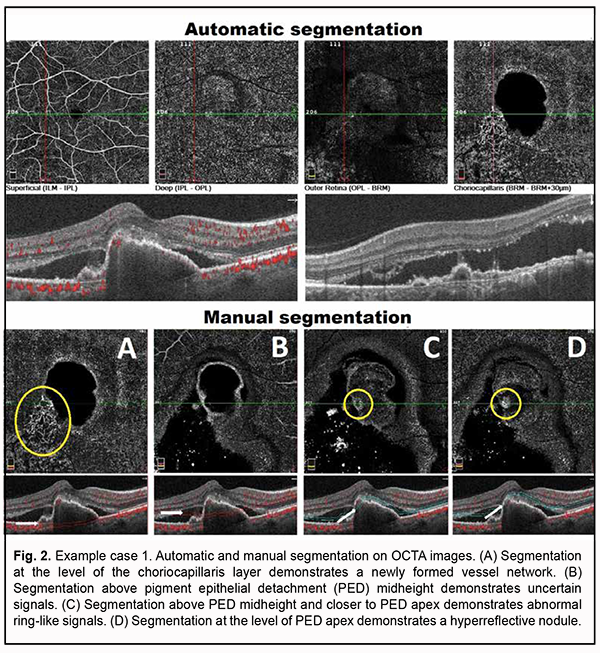
Algorithm for OCTA-based analysis We started the analysis with the assessment of automatic segmentation (Fig. 2). A pathological blood flow signal in the form of a tangled CNV was detected at the choroidal level. In addition, uncertain point-shaped blood flow signals were detected within the outer retina at the area of the supposed polyp. Manual segmentation was used and analysis of the detected symptoms was performed for further analysis (Fig. 2).
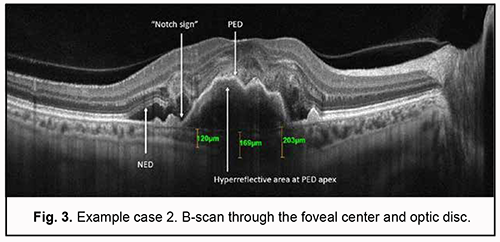
Step 1. An en face slice thickness of 30 µm was selected. In order to assess polypoidal complexes (areas of the PEDs characteristic of PCV), the en face slice was shifted step by step from the choroid towards PED apex. With the slice shifted upward (or to the deeper retinal structures) from the choroid, a neovascular network and then other blood flow signals were detected. Step 2. A single polyp was detected. Step 3. The signals characteristic of PCV were detected: ring-like blood flow signals were detected above PED midheight, and high-intensity blood flow signals, in the nodules, at PED apex. Step 4. Choroidal neovascularization was detected which was adjacent to the vascular complex underneath PED and corresponded to the location of double layer sign in the OCT. The neovascularization had the appearance of a tangled web of fine vessels. Therefore, the OCTA identified the characteristic vascular complexes both within the polyp and within the choroid, making it possible to confirm the diagnosis of late wet active AMD with PCV. Example case 2 A high QRS complex shaped PED characteristic of PCV was found on examination of OCT scans for this example case (Fig. 3). There were such characteristic OCT features strongly suggestive of PCV as a “notch sign” and hyperreflective area at PED apex. Although a DLS was not detected on any macular B-scan, there were additional OCT features (like NED and hyperreflective inclusions in the outer retina) indicating the activity of suspected neovascularization.
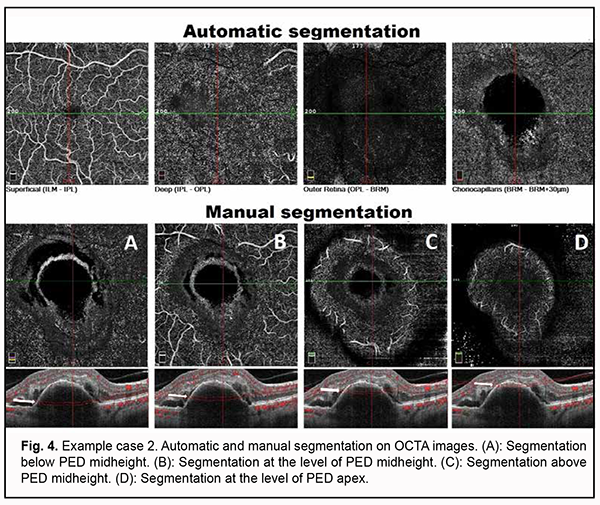
Choroidal assessment did not identify the presence of dilated Haller's layer vessels, and the maximum choroidal thickness at the macula was 203 µm, indicating choroidal thinning. However, given the presence of not only PED, but also two additional OCT features of PCV and signs of activity of subretinal neovascularization, it was neccessary to differentiate between type 1 (occult) subretinal neovascularization and PCV, which required subsequent OCTA imaging. Algorithm for OCTA-based analysis First, the assessment of automatic segmentation was performed (Fig. 4).
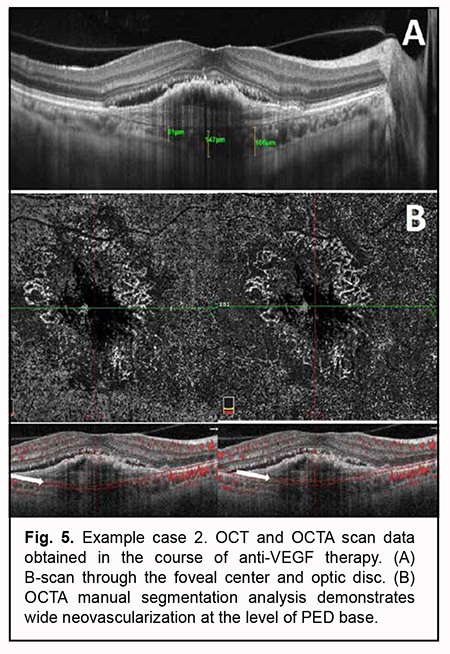
Pathological blood flow signals without the presence of apparent neovascularization were detected at the choroidal level, along the inferior outline of the shadow corresponding to PED. No pathological blood flow was detected within the outer retina and at the level of the retinal superficial and deep vascular plexuses. Manual segmentation assessment was subsequently used for deeper analysis (Fig. 4). Step 1. An en face slice thickness of 30 µm was selected. In order to assess the PED region, the en face slice was shifted step by step from the choroid towards PED apex. Step 2. No abnormal blood flow signal characteristic of a polyp in PCV was detected above PED midheight, at PED apex or at any other segmentation level. Taking into consideration the results of our OCTA scan assessment at various segmentation levels, Step 3 was omitted. Step 4. No apparent neovascular network was detected adjacent to PED. The OCT scan results along with the absence of OCTA signs of PCV or apparent subretinal neovascularization suppose that neovascularization was present within a PED and not visualized due to a high PED and hyperreflective areas of potential hemorrhage. Therefore, the OCTA-based analysis prompted us to reject the presence of PCV and make a diagnosis of late wet active AMD with a type 1 subretinal neovascular membrane. Subsequently, the patient was administered a course of anti-VEGF therapy, and, at the subsequent follow up examination, OCT and OCTA showed a lower PED, which allowed to visualize neovascularization at the level of the choroid, finally confirming the diagnosis for this case (Fig. 5).
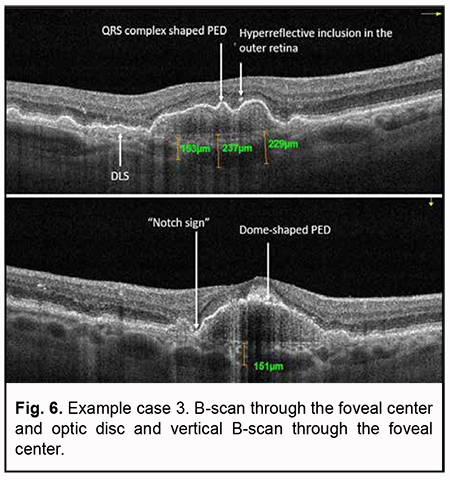
Example case 3 In this example case, OCT also showed a high dome-shaped PED and a “notch sign” characteristic of PCV (Fig. 6). In addition, OCT B-scans showed a QRS complex shaped PED as well as a double-layer sign as a sign of the presence of neovascularization. The signs of activity of suspected subretinal neovascularization noted during scan assessment included cystic cavities and hyperreflective inclusions in the outer retina.
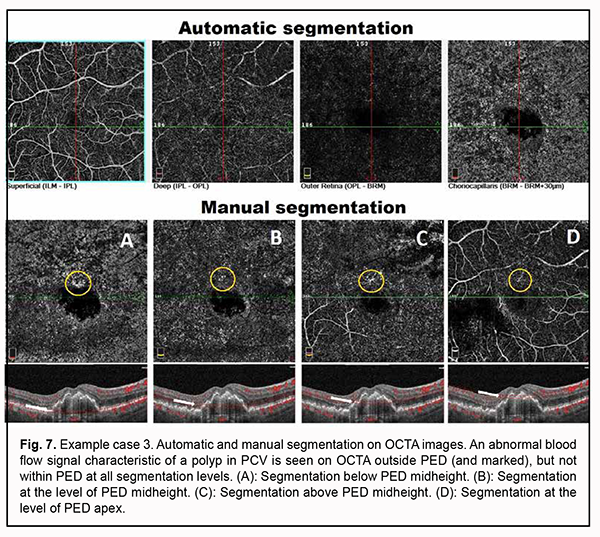
Choroidal thinning was also noted (choroidal thickness at the fovea was 237 µm, and minimum choroidal thickness, 153 µm). Choroidal assessment did not identify the presence of dilated Haller's layer vessels. Given the presence of not only a major feature (i.e., PED), but also two additional OCT features of PCV, it was necessary to perform the OCTA in order to confirm or reject the diagnosis of PCV. Algorithm for OCTA-based analysis First, the assessment of automatic segmentation was performed (Fig. 7).
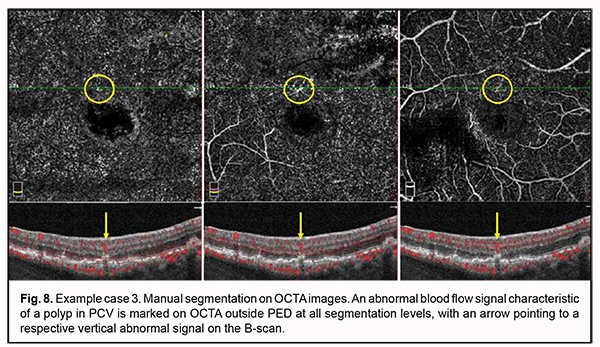
No apparent sign of neovascularization or pathological blood flow was detected on OCTA scans of four segmentation levels. Mildly increased vascular signal at the choroidal level was detected only around the shadow region corresponding to PED. Manual segmentation of OCTA scans was subsequently used for a more accurate assessment (Fig. 7). Step 1. An en face slice thickness of 30 µm was selected and, in order to assess the area of PED, the en face slice was shifted step by step from the choroid towards PED apex. Step 2. No abnormal blood flow signal characteristic of a polyp in PCV was detected on OCTA within PED at any manual segmentation level. Taking into consideration the results of the previous step, Step 3 was omitted. Step 4. No apparent neovascular network was detected adjacent to PED. However, a local pathological signal was detected in the superior parafoveal sector, outside the PED dome at all manual segmentation levels (Fig. 7). With the B-scan section shifted to this region, there was a vertical blood flow signal from PED towards inner retinal layers corresponding to the location of retinal superficial plexus (Fig. 8).
The changes detected by OCTA corresponded to retinochoroidal anastomosis, the presence of which, along with OCT features like uneven PED, a double-layer sign, cystic cavities and hyperreflective inclusions in the outer retina allowed us to make a diagnosis of late wet active AMD with retinal angiomatosis proliferation. The diagnosis of PCV was rejected. In conclusion, when making a diagnostic assessment of suspected PCV in neovascular AMD, a lack of awareness of the clinical OCT and OCTA features of the disease, as well as the similarity in the clinical picture between PCV and other types of neovascularization, may cause misdiagnosis, leading to an inadequate treatment strategy. Our example cases demonstrated that the use of the proposed algorithm for OCTA-based analysis of eyes with suspected PCV allowed to minimize errors in the diagnostic assessment of this pathology.
References 1. Lutsenko NS, Rudycheva OA, Isakova OA, Kyrylova TS. Assessing OCTA changes in morphology and structure of retinal microvascular bed in patients with exudative AMD. J Ophthalmol (Ukraine). 2019;2:7-13. 2. Lutsenko NS, Isakova OA, Rudycheva OA, Kyrylova TS, Unguryan NV. [Macula. Today's diagnostics. (Optical coherent tomography and Optical coherent tomography angiography)]. Zaporizhzhia: Orbita-Yug Agency; 2019. Ukrainian. 3. Shaimov TB, Panova IP, Shaimov RB, Shaimov TA, Fomin AV. [Optical coherence tomography-angiography in the diagnosis of neovascular age-related macular degeneration]. Bull of Ophthalmology. 2015 Sep-Oct;131(5):4-13. Russian. 4. Lumbroso B, Huang D, Souied EH, Savastano MC, Jia Y, Rispoli M. Understanding OCT Angiography from Pathophysiology to Clinical Imaging. 1st ed. New Delhi: Jaypee Brothers medical Publishers; 2019. 5. Borrelli E, Battista M, Gelormini F. Sacconi R, Querques L, Vella G, Vigano C, Bandello F, Querques G. Rate of misdiagnosis and clinical usefulness of the correct diagnosis in exudative neovascular maculopathy secondary to AMD versus pachychoroid disease. Sci Rep. 2020;10:20344. 6. Lauermann JL, Woetzel AK, Treder M, Alnawaiseh M, Clemens CR, Eter N, Alten F. Prevalences of segmentation errors and motion artifacts in OCT-angiography differ among retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2018 Oct;256(10):1807-16. 7. Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen S, Chen Y, Freund KB, Gomi F, Koh AH, Lee WK, Wong TY. Polypoidal Choroidal Vasculopathy. Definition, Pathogenesis, Diagnosis, and Management. Ophthalmology. 2018 May;125(5):708-724. 8. Shaymov TB, Panova YE, Shaymova VA. [Differential diagnostic criteria in the diagnosis of polypoid choroidal vasculopathy as a variant of the course of the neovascular stage of age-related macular degeneration. Modern technologies in ophthalmology]. Russ ophthalmol onl [Internet]. 2017 Mar 1;1(14):335–9. Russian. 9. Tomiyasu T, Nozaki M, Yoshida M, Ogura Y. Characteristics of polypoidal choroidal vasculopathy evaluated by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016 July;57(9):324-30. 10. Shaymov TB, Panova YE, Shaymova VA. [Algorithm for non-invasive clinical and instrumental diagnostics of polypoid choroidal vasculopathy. Modern technologies in ophthalmology]. Russ ophthalmol onl [Internet]. 2018 Jun 14;1(21):402-407. Russian. 11. Yang J, Yuan M, Wang E, Xia S, Chen Y. Noninvasive multimodal imaging in diagnosing polypoidal choroidal vasculopathy. BMC Ophthalmol. 2019 Nov 16;19(1):229-36. 12. National Institute for Health and Care Excellence. Age-related macular degeneration: diagnosis and management [Internet]. London: NICE; 2018 Jan;Clinical guideline (NG82):244. Available from: https://www.nice.org.uk/guidance/ng82/evidence/full-guideline-pdf-170036...
13. Lutsenko NS, Rudycheva OA, Isakova OA, Kyrylova TS. How to avoid mistakes in the diagnosis of polypoid choroidal vasculopathy in patients with age-related macular degeneration (part one). J Ophthalmol (Ukraine). 2021;4: 32-38.
|