J.ophthalmol.(Ukraine).2021;6:56-63.
|
http://doi.org/10.31288/oftalmolzh202165663 Received: 15 July 2021; Published on-line: 21 December 2021 Sympathetic uveitis in children: a case report and literature review N. F. Bobrova, S. A. Tronina, O. V. Ivanytska, L. M. Velychko, O. V. Artiomov, A. V. Bogdanova SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: filatov.detskoe7@gmail.com TO CITE THIS ARTICLE: Bobrova NF, Tronina SA, Ivanytska OV, Velychko LM, Artiomov OV, Bogdanova AV. Sympathetic uveitis in children: a case report and literature review. J.ophthalmol.(Ukraine). 2021;6:56-63. http://doi.org/10.31288/oftalmolzh202165663 Background: Sympathetic ophthalmia is a rare autoimmune disease that presents as a bilateral granulomatous uveitis following a traumatic event or, less commonly, uniocular surgery. Purpose: To describe immunohistological and histomorphological data of a child who developed sympathetical ophthalmia in a healthy eye after a severe penetrating corneoscleral injury with contusion component. Material and Methods: We present clinical observation data of a boy of 6.5 years who developed sympathetic ophthalmia in the left eye 1.5 months after a penetrating ocular injury caused by a tree branch resulted in a severe corneoscleral laceration in the right eye. Types of treatment and data of clinical, imaging (ultrasound scanning and spectral-domain optical coherence tomography (SD-OCT)), pathohistological and immunological studies were described and reviewed. Conclusion: A high index of clinical suspicion and careful monitoring of the state of not only a traumatized eye, but also the fellow eye both in the early and late period after traumatic event are vital to ensure identification of initial signs of sympathetic inflammation (photophobia and tearing) and taking prompt adequate measures for the prevention of further development of sympathetic inflammation. The use of current imaging modalities (including ultrasound scanning and especially SD-OCT of the retina and optic nerve) enables early documentation of the initial signs of sympathetic inflammation. Further research is warranted to develop the targeted treatment methods that would take into account the components of the immunopathogenesis Keywords: sympathetic ophthalmia, trauma, children
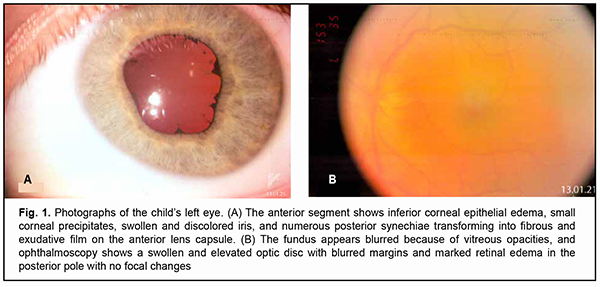
Sympathetic ophthalmia is a rare autoimmune disease that presents as a bilateral granulomatous uveitis following a traumatic event or, less commonly, uniocular surgery. The injured or operated eye is the exciting eye and the contralateral eye is the sympathizing eye. The term “sympathetic ophthalmia” ( inflammation of one eye due to injury or disease of the other eye) is derived from the Greek words “sympathes” (meaning “having a fellow feeling, affected by like feelings”) and “ophthalmos” (meaning “the eye”). The cause of sympathetic ophthalmia is not completely known. Many doctors think that it is due to a malfunction the body’s immune system that causes the body to attack the uninjured uveal tract. Advances in methods of initial surgical debridement of penetrating injuries and increased use of corticosteroids in medical management of patients with sympathetic ophthalmia have contributed to a substantial decrease in the incidence of the disease, which currently ranges from 0.2% to 0.4% [1]. The disease is more common in young people. In addition, more than half of young patients with sympathetic ophthalmia are children and adolescents under 15 years of age [2], which may be explained by the relative incompetence of the immature immune system. In recent time, the opinion has emerged that sympathetic ophthalmia is a vanishing disease, which Arkhipova [3] believes to be wrong and even dangerous. The disease is of special importance because both eyes are involved in the pathological process and treatment outcomes are often very poor. Given the rarity of the disease and the severity of the clinical course and unsatisfactory outcomes, we would like to share our experience with a case of sympathetic ophthalmia. The purpose of the study was to describe immunohistological and histomorphological data of a child who developed sympathetical ophthalmia in a healthy eye after a severe penetrating corneoscleral laceration with contusion component. Material and Methods On October 22, 2020, a boy of 6.5 years was urgently hospitalized to the Department of Pediatric Eye Disorders, the Filatov institute, for an extensive penetrating corneoscleral laceration with prolapse of the ocular contents, hyphema and vitreous hemorrhage in the right eye and right upper eyelid laceration following severe tree branch trauma. Urgent surgical treatment was performed which included initial surgical debridement of the corneoscleral laceration and right upper eyelid laceration. On admission, visual acuity was zero in the right eye and 1.0 in the left eye. Postoperatively, the patient received an intensive course of infusion antibacterial, anti-inflammatory, and hemostatic therapy. In addition, he received a course of parabulbar injections of an antibiotic and corticosteroid beginning from day 4 after surgery. He was discharged in satisfactory clinical condition to have his treatment continued at his locality. At discharge, visual acuity was zero in the right eye and 1.0 in the left eye. In the middle of December of 2020, the child’s mother noticed that his healthy left eye became red and photophobic. The boy underwent an examination of the eye at the local health center, was diagnosed with conjunctivitis, and had antibacterial eye drops prescribed for this condition. On control examination, keratic precipitates were seen on the corneal endothelium of the left eye, and the child was referred to our institution for suspected sympathetic ophthalmia. In order to clarify the state of the traumatized eye and the fellow eye, the following studies were conducted along with a standard ophthalmic examination: ocular ultrasound scanning (Aviso; Quantel Medical, Clermont-Ferrand, France), spectral-domain optical coherence tomography (SD-OCT Spectralis, Heidelberg Engineering, GmbH, Dossenheim, Germany) of the retina and optic nerve, electrophysiological study (electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance, visually evoked potentials (VEPs) were recorded using a RetiScan unit (Roland Consult, Germany), immunological study (expression of lymphocyte activation marker CD 54 (ICAM-1)), and histomorphological study of the enucleated right eye. Results After repeat urgent hospitalization on January 5, 2021, examination findings were as follows. There was a vertical scar in the medial third of the right upper eyelid. In addition, the palpebral fissure was narrow, the eye was photophobic and esophoric OD. The right globe was somewhat reduced in size. Slit-lamp examination found an intense pericorneal and conjunctival injection. There was a vertical corneal scar at the 12 o’clock to 8 o’clock position, with adhesion to sclera at the 8 o’clock position. The anterior chamber was extremely shallow and aqueous humor transparent. The iris pattern was smoothed. The pupil was occluded by scar tissue. No fundus reflex was seen. There was severe hypotony. The left eye was photophobic and had a narrow palpebral fissure. Slit-lamp examination found a pericorneal and conjunctival injection, mild corneal edema, edema of the inferior corneal endothelium, and small keratic precipitates OS. The anterior chamber was moderately deep, with some opacification of the aqueous humor. The iris was edematous and discolored. There were numerous posterior synechiae transforming into fibrous and exudative film on the anterior lens capsule (Fig. 1A). Fibrinous exudate was seen in the anterior third of the vitreous and was more marked in the superior outer segment. The fundus reflex was diminished. Fundus examination showed an elevated optic disc with blurred margins, a posterior segment edema, and no focal lesions OS. The IOP was 18 mm Hg and uncorrected visual acuity, 0.4 OS (Fig. 1B).
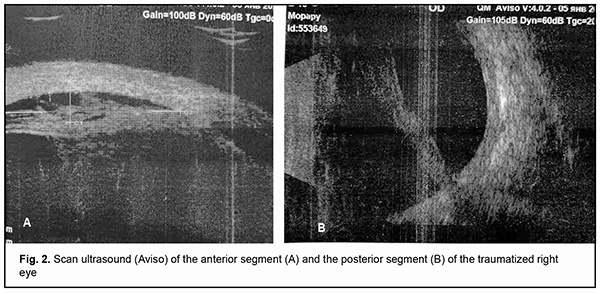
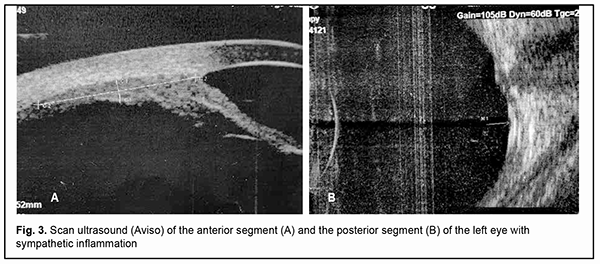
Ultrasound scanning showed an inhomogenous cornea which was non-uniformly thickened with a maximum thickness of up 1.1 mm in the center. The anterior chamber was shallow (0.8 mm). There was a moderately echogenic fibrous substrate as thick as 2.2 mm at the site of the lens. A circumferential ciliochoroidal detachment as high as 1.2 mm and as long as 7.5 mm as well as a high closed funnel-shaped retinal detachment was noted (Fig. 2A,B). The anterior chamber was moderately shallow (3.4 mm). An anechogenic lens was of 3.3-mm thickness. The ciliary body was unevenly circumferentially thickened along 0.6 mm with a maximum thickness of 0.7 mm at the pars plana ciliaris. Hypoechogenic floaters were noted at the posterior vitreous. The retina appeared attached (Fig. 3 A, B). Ultrasound biometry showed axial length of 21.12 mm OD and 23.14 mm OS.
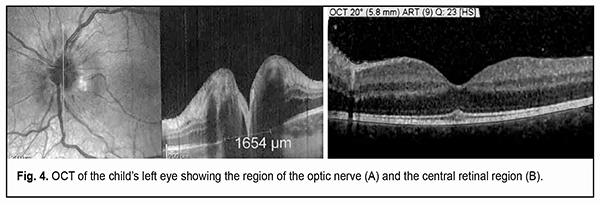
The electrophysiological study found no electrical sensitivity of the optic nerve for phosphene OD, and the electrical sensitivity threshold OS was 67 µA. There was minimal normal bioelectrical activity of the optic nerve OS as assessed by VEP. The patient was diagnosed with sympathetic uveitis OS, and received an intensive course of infusion antibacterial and anti-inflammatory therapy with topical and systemic corticosteroid use and physiotherapy OS. At the day of admission, a decision was made on urgent enucleation of the right sympathizing blind eye and application of the orbital implant for the formation of locomotor stump. Further studies of the left eye were conducted after enucleation of the right eye. SD-OCT showed changes in the optic nerve OS, with marked retinal nerve fiber layer thickening in all optic disc segments evaluated, neural tissue thickening at the neural ring, and elevated nasal optic disc sector and the superior and inferior optic disc poles (Fig. 4A). Retinal analysis showed a significant retinal thickening at the macula, and, according to the Thickness Map Single Exam Report, the Total Retinal Volume at a 5.8-mm diameter zone was 10.59 mm2.
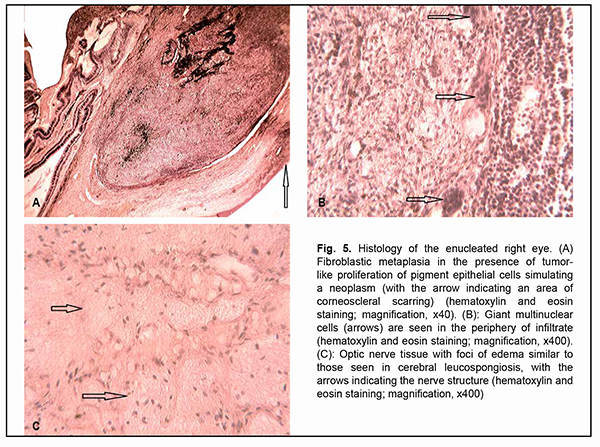
In addition, this retinal thickening was associated with diffuse retinal edema, which was most apparent in the neuronal layers, especially, the outer nuclear layer, with no focal changes (Fig. 4B). Although Dalen-Fuchs nodules were not found at retinal pigment epithelium (RPE) level, microstructural changes were noted in the peripapillary retina, at the level of the outer nuclear layer, in its myoidal area, in the form of small inner retinal confluent hyporeflective spaces of different size, with mild deformation of the outer plexiform layer above these spaces. Hyperreflective inclusions were found in practically each of these void hyporeflective spaces, which may indicate partial lysis of the cells with deformed membrane. Vertical hypodense areas of the inner nuclear layer were seen adjacent to optic disc margin, indicating the involvement of the brain layer of the retina in the pathological process, which was confirmed by the thickening of both the ganglion cell layer and the retinal nerve fiber layer. The immunological study found high expressions of lymphocyte and neutrophil activation markers. Particularly, CD54 (intercellular adhesion molecule-1, ICAM-1) expression was 34% compared with a normal value of 9.89% ± 4.3%, CD38 expression was 37% compared with a normal value of 10.1% ± 3.2%, and CD95 expression was 22% compared with a normal value of 12.3% ± 4.45%. In addition, the expression of CD15, a neutrophil activation marker, was as high as 22.0% compared with a normal value of 12.0 ± 4.0%. Histomorphological study of the enucleated right eye found a severely impaired topographic relationship between the inner coats of the eye in the presence of inflammation and reparative post-traumatic proliferation. Thus, in the anterior segment of the eye, we noted the growth of neovascularized fibrous connective tissue at the area of corneoscleral scarring, with the formation of a clump of fragments of both lens substance and a glass-like lens epithelial membrane (Fig. 5A). The choroid was seen infiltrated mostly with lymphocyte type cells and minor amount of plasma and epithelioid cells practically from the optic nerve to the ora serrata. Aside of the above-mentioned inflammatory cells, giant multinuclear cells resembling foreign bodies were present in the above clump (Fig. 5B). Aside from the changes in the inner coats of the eye, we noted changes in the extrabulbar portion of the optic nerve in the form of severe congestion and stasis of the vessels of the perineural optic sheaths with swelling of the optic nerve tissue, which appeared histologically as increased gaps between neuroglial cells similar to those seen in cerebral leucospongiosis (Fig. 5C).
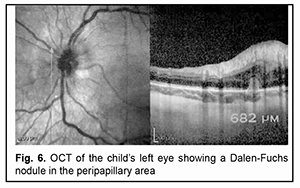
The patient experienced sustained improvement in inflammation and vitreous exudate as well as an improvement in visual acuity to 0.85 with his initial course of therapy. However, on day 10 of the treatment, exacerbation of uveitis was noted in the form of the appearance of new synechiae and increased exudate in the vitreous developing in the presence of active therapy and in the absence of any provoking factors. In this connection, in addition to the continued systemic therapy, cyclosporine was administered orally at an initial dose of 2 mg/kg/day with monitoring of tolerance and an increase in dose to 2 mg/kg/day at day 3 of cyclosporine treatment. After administration of systemic cytostatic therapy, further improvement was noted in the form of complete resolution of keratic precipitates, partial resolution of strong posterior synechiae, exudative film on the anterior lens surface and vitreous opacities. Repeat ocular ultrasound scanning showed a decrease in the thickening of the ciliary body, with a decrease in extension to 3.5 mm, and in height to 3.0 mm. On day 24 after hospitalization, the child was discharged in satisfactory clinical condition, with visual acuity 1.0 OS, to have his treatment (systemic methylprednisolon 4 mg/day and cyclosporine 75 mg/day) continued on the outpatient basis. Since re-exacerbation of uveitis developed in 1.5 months, the child was re-hospitalized. At admission, there was moderate pericorneal and conjunctival injection as well as mild blepharospasm. In addition, there were corneal endothelial edema and numerous wide posterior synechae. The opacification of the lens and vitreous increased compared to the condition at discharge, which resulted in a decrease in visual acuity to 0.12. Ocular ultrasound scanning revealed diffuse thickening of the ciliary body and peripheral choroid. In addition, above and adjacent to the ciliary body, there were hypoechogenic fibrous structures in the form of a 0.4-mm wide and 5-mm long layer. Moreover, there was 0.7-mm optic disc elevation as well as increased peripapillary retinal nerve fiber layer thickness. As there was re-exacerbation of sympathetic ophthalmia, parabulbar dexamethasone and subtenon kenalog injections were additionally administered to the patient, and a dose of cyclosporine was increased to 100 mg/day. This resulted in the relief of exacerbation improved, with an improvement in visual acuity to 0.6. On re-examination two months later, no symptoms of acute uveitis were noted in the sympathetic eye, but lens opacities worsened, resulting in a decrease in visual functions. The patient received an additional anti-relapse course of anti-inflammatory and pro-resolving therapy, which resulted in an improvement in visual acuity from 0.17 to 0.6. Examinations performed at subsequent follow-up time points found the inflammation control to be effective, which enabled a gradual daily dose reduction to 2 mg for systemic corticosteroids and 50 mg for immunosuppressive agents. It is interesting that retinal SD-OCT found Dalen-Fuchs nodules five months after the development of sympathetic ophthalmia (Fig. 6).
Discussion Although sympathetic ophthalmia was first recognized by Hippocrates as early as the fifth century BC, it is poorly understood because the condition is rare, with the incidence ranging from 0.2 to 0.4% after penetrating ocular injuries and 0.01 to 0.06% after intraocular surgery [2]. It was first described and named by Mackenzie in 1840. However, only in 1905 Fuchs [4] first reported on the ocular histopathology of sympathetic ophthalmia, and described the infiltration of the uvea (particularly, of the choroid), with the formation of nodules below the retinal pigment epithelium. These aggregates had been noted earlier by Dalen, a contemporary of Fuchs, and were later named the Dalen-Fuchs nodules. The time from ocular injury to onset of SO varies greatly, ranging from two weeks to 66 years [5]. Williams and colleagues [6] reported on a case of sympathetic ophthalmia presenting 5 days after penetrating injury, but 90% of the cases (including the case reported herein) occur within 1 year after injury to the exciting eye [7-9]. Epidemiology. It is generally agreed that the inciting event behind sympathetic ophthalmia is penetrating globe injury [3, 10]. Recent studies [3, 11, 12], however, demonstrated, that the main current risk is eye surgery, particularly retinal surgery [1, 21, 29]. Although incidence of sympathetic ophthalmia has declined in the recent past, prevalence of ocular surgery is increasing and necessitates careful monitoring of changing disease incidence [7]. In addition, the cases of sympathetic ophthalmia developing following non-perforating eye surgery, ruthenium plaque brachytherapy, cyclodestructive procedures, and proton beam irradiation have been reported [13-16]. Moreover, corneoscleral lesions, prolonged iridocyclitis and initial subatrophy of the traumatized eye are common in children with sympathetic ophthalmia [2], and were present in the case reported herein. As practice shows, in children, the disease is preceded by sympathetic irritation, which is manifested in the form of photophobia, tearing and pericorneal injection in the healthy fellow eye. These symptoms may be manifested or increased by stimulation of the healthy eye or traumatized eye by flashing light. Obviously, in the case reported herein, the disease also was preceded by sympathetic irritation which was misdiagnosed as conjunctivitis. It was a delay in both establishing the correct diagnosis and taking prompt treatment measures to prevent sympathetic inflammation that triggered a cascade of autoimmune processes. Symptoms of anterior uveitis with the presence of mutton-fat keratic precipitates can be seen in 15% of patients with sympathetic ophthalmia. Most commonly (50–85 %), sympathetic ophthalmia presents as panuveitis, with the involvement of both the anterior and posterior uvea, formation of typical Dalen-Fuchs nodules the fundus, and optic nerve edema, sometimes exudative retinal detachment, ultrasonographic evidence of thickened choroid, and OCT and fundus fluorescein angiography (FA) evidence of changes in the retinal pigment epithelium [2,3, 7, 9]. In the pediatric case of sympathetic ophthalmia reported herein, there were no typical mutton-fat precipitates on the endothelium or Dalen-Fuchs nodules in the presence of full-blown panuveitis. In addition, it is noteworthy that the histopathology found signs of diffuse non-specific chronic granulomatous inflammation, but not typical Dalen-Fuchs nodules in the enucleated eye. Lubin and colleagues [17] also noted that although these aggregates are pathognomonic for sympathetic ophthalmia, they are found only in 25-30% of the enucleated eyes. Chu and Foster [7] and Castiblanco and Adelman [18] stressed on the importance of ocular imaging techniques in the diagnostic evaluation of sympathetic ophthalmia. Thus, fundus photography may be helpful in detecting yellowish-white Dalen-Fuchs nodules and choroidal granulomatous infiltrates, retinal vasculitis, serous retinal detachment, and optic nerve swelling. Fluorescein angiography can show multiple hypofluorescing and hyperfluorescing spots at the RPE level in the early venous phase, Dalen-Fuchs nodules can sometimes be visualized as early areas of hypofluorescence, and less commonly, retinal vasculitis can be observed in late staining [19]; sometimes the optic disc may also stain [10]. Indocyanine green angiography can show areas of hypofluorescence where subretinal lesions are located, making it a useful adjunct to confirm diagnosis as well as a tool for monitoring response to treatment [18]. However, the use of contrast angiography is limited in pediatric patients (especially in children of 3 to 7 years) due to the potential for developing apparent allergic reactions. The use of OCT has no such limitation, and the method is used for detecting retinal thickening in cases of sympathetic ophthalmia, which allows identifying serous retinal detachment and intreretinal edema, and detecting the destruction of the RPE as well as disorganization of the internal retinal layers. Chan and colleagues [20] used serial OCT images for the quantitative monitoring of the status of the retina and demonstration of the progression or relief of serous retinal detachment, which confirms that the OCT technique is a reliable means for monitoring sympathetic ophthalmia treatment response. The extent of choroidal thickening by inflammatory infiltrate in sympathetic ophthalmia can be visualized through B-scan ultrasonography and can be useful in differentiating sympathetic ophthalmia from bilateral phacoanaphylactic endophthalmitis [18]. In the case reported herein, SD-OCT not only allowed to detect structural changes in the retina and optic disc, but also was more sensitive for detecting choroidal thickening than ultrasound scanning. In addition, in this case, SD-OCT confirmed the absence of Dalen-Fuchs nodules in the development of neuroretinitis as a form of sympathetic ophthalmia. However, diffuse retinal thickening at the macula, peripapillary retinal thickening, and RNFL thickening, and microstructural changes in the inner and outer nuclear layers with deformation of the outer plexiform layer demonstrate the presence of neuroretinitis as a form of sympathetic ophthalmia. The original hypothesis proposed by Mackenzie and his contemporaries in the nineteenth century suggested the bilateral impact of sympathetic ophthalmia to be the result of inflammation in the injured eye that propagated along the optic nerve and chiasma to the contralateral eye. In the early twentieth century, it was proposed that the disease was a hypersensitivity reaction and that the antigen responsible was melanin [21]. The strong correlation between uveal injury and sympathetic ophthalmia also led some to suspect the pathogenic role of the uvea, citing the presence of antiuveal antibodies in a large percentage of patients with sympathetic ophthalmia. The most convincing theory thus far points to the role of cell-mediated immune response to antigens from the retinal photoreceptor layer. While the particular antigen is yet to be determined, putative retinal antigens include retinal soluble antigen (S-antigen) [22], rhodopsin [23], interphotoreceptor retinoid-binding protein [24], and recoverin [25]. Genetic predisposition to the development of sympathetic ophthalmia has also been proposed, with particular focus on human leukocyte antigen (HLA) [26]. Enucleation of the sympathizing eye is a classical and still relevant approach to the treatment of sympathetic ophthalmia. Some authors considered indications for enucleation as early as 10-14 days after traumatic event, before the development of clinical signs of sympathetic ophthalmia in the healthy fellow eye, especially in the presence of substantial damage to the eye [27, 28]. Their opponents noted that (a) sympathetic ophthalmia can develop as early as 5 days after traumatic event and (b) early enucleation may have no impact on the outcome of treatment of the sympathetic eye, because the visual outcome for the affected eye may be better than that for the sympathetic eye [19, 29]. The questions regarding the choice between enucleation and evisceration for the traumatized have been discussed. The main conclusion was that adherence to the procedure of evisceration and diligent removal of all elements of the choroid in combination with neurotomy does not result in an increase in the incidence of sympathetic ophthalmia [30, 31]. Nevertheless, the classic enucleation is still the preferred method of eye removal for most surgeons due to potential problems with the removal of the choroid incarcerated in scleral wounds in the course of evisceration. The enucleation of the traumatized blind eye in the case reported herein was reasonable because there were no prospects for its functional restoration, and the choice of evisceration was associated with a risk of incarceration of choroidal fragments in an extensive corneoscleral wound. Anti-inflammatory corticosteroids are the first-line therapy for sympathetic ophthalmia and may be given topically and systemically. In cases refractory to corticosteroids and in patients with significant systemic side effects, immunosuppressive therapy that combines systemic corticosteroids and other immunosuppressive agents such as cyclosporine or azathioprine can improve prognosis [7]. In the case reported herein, we had to favor this way of intensification of treatment due to acute panuveitis (in the form of appearance of new synechia and an increased exudation into the vitreous) in the sympathetic eye in the presence of systemic corticosteroid treatment. Cyclosporine as an adjunctive treatment resulted in prompt relief of the above phenomena and, in the presence of careful monitoring of the child’s blood characteristics and general condition was not accompanied by any side effects. However, this massive systemic anti-inflammatory and cytostatic therapy failed to provide complete control of the autoimmune process, which resulted in recurrent sympathetic ophthalmia in the presence of continued treatment. An additional increase in topical anti-inflammatory corticosteroid therapy was effective for the relief of recurrent sympathetic ophthalmia. Numerous humoral mediators are involved in the regulation of immune mechanisms, and impairment in the regulation is believed to play a major role in the development of sympathetic ophthalmia. Cytokines, the low molecular weight proteins that mediate intercellular communication, are important humoral mediators. The type of immune response depends on the cytokine ratio leading to predominant activation of T cell sets (mainly, CD4). A high expression of CD54, a functional biomarker of inflammation and co-stimulator of lymphocyte activation, was observed in our case. ICAM-1is expressed on the cell surface, activated in response to mediators of inflammation (e.g., various anti-inflammatory cytokines), and important for lymphocyte migration from blood through vascular endothelium to the ocular tissue. During inflammation, CD54 expression is activated by anti-inflammatory cytokines, especially (tumor necrosis factor) TNF-a, on the endothelial cells of not only blood vessels, but also in the ocular tissue. ICAM-1 is believed to play a special role in the development of autoimmune disease. This intercellular adhesion molecule can significantly accelerate the progression and generalization of inflammatory processes [32]. Disruption of the protective mechanism (with abnormal expression of the molecules influencing the cells of the immune system on the surface of intraocular cells, and synthesis of factors inhibiting T cell proliferation and secretion of anti-inflammatory cytokines) is an important factor in the development of sympathetic inflammation and progression of immune damage to ocular structures. Conclusion In spite of advances in eye microsurgery and the use of efficacious medications in the postoperative period, sympathetic ophthalmia still may occur following pediatric eye injury. A high index of clinical suspicion and careful monitoring of the state of not only a traumatized eye, but also the fellow eye both in the early and late period after traumatic event are vital to ensure identification of initial signs of sympathetic inflammation (photophobia and tearing) and taking prompt adequate measures for the prevention of further development of sympathetic inflammation. The use of current imaging modalities (including ultrasound scanning and SD-OCT of the retina and optic nerve) enables early documentation of the initial signs of sympathetic inflammation. With regard to the treatment of sympathetic ophthalmia, although the most common approach is still classical prompt enucleation of the inciting eye which is usually blind and atrophic, combined with single-stage systemic anti-inflammatory and immunomodulatory therapy, further research is warranted to develop the targeted methods that would take into account the components of the immunopathogenesis.
References 1.Kumar K., Mathai A., Murthy S.I. Sympathetic ophthalmia in pediatric age group: clinical features and challenges in management in a tertiary center in Southern India. Ocul Immunol Inflamm. 2014 Oct;22(5):367-72. 2.Bobrova NF. [Reconstructive eye surgery in children]. Odessa: Phoenix; 2013. Russian. 3.Arkhipova LT. [On the frequency of sympathetic ophthalmia. Myth and reality]. Russian Ophthalmological Journal. 2016; 9 (3): 95-100. Russian. 4.Albert DM, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989; 34 (1):1–14. 5.Zaharia MA, Lamarche J, Laurin M. Sympathetic uveitis 66 years after injury. Can J Ophthalmol. 1984 Aug;19(5):240-3. 6.Williams AM, Shepler AM, Chu ChT, Nischal KK. Sympathetic ophthalmia presenting 5 days after penetrating injury. Am J Ophthalmol Case Rep. 2020 Jul 9;19:100816. doi: 10.1016/j.ajoc.2020.100816. eCollection 2020 Sep. 7.Chu XK, Chan Chi-Chao. Sympathetic ophthalmia: to the twenty-first century and beyond. J Ophthalmic Inflamm Infect. 2013 Jun 1;3(1):49. 8.Goto H, Rao NA. Sympathetic ophthalmia and Vogt-Koyanagi-Harada syndrome. Int Ophthalmol Clin. Fall 1990;30(4):279-85. 9.Marak Jr GE. Sympathetic uveitis. In: Yanoff M, Duker JS, editors. 2nd ed. Mosby: St.Louis; 2004: 1205–8. 10.Chu DS, Foster CS. Sympathetic ophthalmia. Int Ophthalmol Clin. Summer 2002;42(3):179-85. 11.Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol. 2000 Mar;84(3):259-63. 12.Su DH, Chee SP. Sympathetic ophthalmia in Singapore: new trends in an old disease. Graef Arch Clin Exp. 2006 Feb;244(2):243-7. 13.Ahmad N, Soong TK, Salvi S, Rudle PA, Rennie IG. Sympathetic ophthalmia after ruthenium plaque brachytherapy. Br J Ophthalmol. 2007; 91 (3):399–401. 14.Arevalo JF, Garcia RA, Al-Dhibi HA, Sanchez JG, Suarez-Tata L. Update on sympathetic ophthalmia. Middle East Afr J Ophthalmol. 2012 Jan-Mar; 19(1): 13–21. 15.Bechrakis NE, Muller-Stolzenburg NW, Helbig H, Foerster MH. Sympathetic ophthalmia following laser cyclocoagulation. Arch Ophthalmol. 1994 Jan;112(1):80-4. 16.Brour J, Desjardins L, Lehoang P, Bodaghi B, Lumbroso-Lerouic L, Dendale R, Cassoux N. Sympathetic ophthalmia after proton beam irradiation for choroidal melanoma. Ocul Immunol Inflamm. 2012; 20 (4):273–6. 17.Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913–1978). Ophthalmology. 1980 Feb;87(2):109-21. 18.Castiblanco C, Adelman RA. Imaging for sympathetic ophthalmia: impact on the diagnosis and management. Int Ophthalmol Clin. Fall 2012;52(4):173-81. 19.Damico FM, Kiss S, Young LH. Sympathetic ophthalmia. Semin Ophthalmol. Jul-Sep 2005;20(3):191-7. 20.Chan RV, Seiff BD, Lincoff HA, Coleman DJ. Rapid recovery of sympathetic ophthalmia with treatment augmented by intravitreal steroids. Retina. 2006 Feb;26(2):243-7. 21.Elschnig A. Studies on sympathetic ophthalmia, II: the antigenic effect of eye pigments. Graef Arch Clin Exp. 1910; 3: 365–456. 22.de Kozak Y, Sakai J, Thillaye B, Faure JP. S antigen-induced experimental autoimmune uveo-retinitis in rats. Curr Eye Res. 1981;1(6):327-37. 23.Schalken JJ, Winkens HJ, Van Vugt AH, De Grip WJ, Broekhuyse RM. Rhodopsin-induced experimental autoimmune uveoretinitis in monkeys. Br J Ophthalmol. 1989 Mar;73(3):168-72. 25.Gery I, Chanaud NP 3rd, Anglade E. Recoverin is highly uveitogenic in Lewis rats. Invest Ophth Vis Sci. 1994 Jul;35(8):3342-5. 26.Shindo Y, Ohno S, Usui M, Ideta H, Harada K, Masuda H, Inoko H. Immunogenetic study of sympathetic ophthalmia. Tissue Antigens. 1997 Feb;49(2):111-5. 27.Bilyk JR. Enucleation, evisceration, and sympathetic ophthalmia. Curr Opin Ophthalmol. 2000 Oct;11(5):372-86. 28.Chaithanyaa N, Devireddy SK, Kishore Kumar RV, Gali RS, Aneja V. Sympathetic ophthalmia: a review of literature. Oral Surg Oral Med Oeal pathol Oral Radiol. 2012 Feb;113(2):172-6. 29.Galor A, Davis JL, Flynn HW Jr, Feuer WJ, Dubovy SR, Setlur V, et al. Sympathetic ophthalmia: incidence of ocular complications and vision loss in the sympathizing eye. Am J Ophthalmol. 2009 Nov;148(5):704-710.e2. 30.Phan LT, Hwang TN, McCulley TJ. Evisceration in the modern age. Middle East Afr J Ophthalmol. 2012 Jan-Mar; 19(1): 24–33. 31.du Toit N, Motala MI, Richards J, Murray AD, Maitra S. The risk of sympathetic ophthalmia following evisceration for penetrating eye injuries at Groote Schuur Hospital. Br J Ophthalmol. 2008 Jan;92(1):61-3. 32.Khramenko NI, Gaidamaka TB, Drozhzhyna GI, Velychko LN, Bogdanova AV. ICAM-1 expression on blood lymphocytes in patients with stromal herpes keratitis at different periods of disease. J Ophthalmol (Ukraine). 2020; 3: 23-8. Russian. Conflict of Interest: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|