J.ophthalmol.(Ukraine).2022;2:27-31.
|
http://doi.org/10.31288/oftalmolzh202222731 Received: 15 February 2021; Published on-line: 30 April 2022 Comparing the efficacy of non-penetrating deep sclerectomy combined with endotrabeculectomy with that of trabeculectomy I. Ia. Novytskyy, O. V. Levytska Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) E-mail: Inovytskyy@gmail.com TO CITE THIS ARTICLE: Novytskyy IIa, Levytska OV. Comparing the efficacy of non-penetrating deep sclerectomy combined with endotrabeculectomy with that of trabeculectomy. J.ophthalmol.(Ukraine).2022;2:27-31. http://doi.org/10.31288/oftalmolzh202222731 Purpose: To compare the clinical efficacy of non-penetrating deep sclerectomy (NPDS) combined with ab interno endotrabeculectomy (ETE) with that of penetrating trabeculectomy for primary open-angle glaucoma (POAG). Material and Methods: Twenty-five patients (25 eyes) that received surgery for POAG were included in the study and divided into two groups based on the type of surgery received: trabeculectomy (13 patients) and NPDS combined with ETE (12 patients). Results: There was a significant difference (a) between preoperative and any postoperative intraocular pressure (IOP) levels, and (b) between preoperative and postoperative mean numbers of ocular hypotensive medications used for either group at any time point of the 12-month follow-up (р < 0.05). In addition, there was no significant difference in IOP between groups (р > 0.05) at any time point. Moreover, there was no significant difference in the number of ocular hypotensive medications used between the groups at any time point. Conclusion: Not only EDE combined with NPDS has at least the same ocular hypotensive effect as that of trabeculectomy for POAG, but it is also advantageous in a low incidence of severe postoperative complications, which may make it a procedure of choice for POAG. Keywords: endotrabeculectomy, non-penetrating deep sclerectomy, intraocular pressure, topical ocular hypotensive medications, trabeculectomy
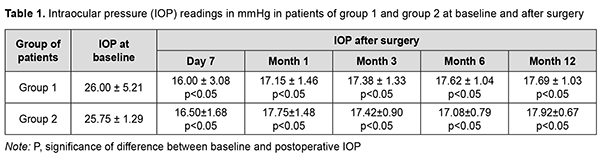
Introduction Glaucoma is a group of progressive optic neuropathies that have in common characteristic optic nerve head changes, loss of retinal ganglion cells and visual field defects. Among the large family of glaucomas, primary open-angle glaucoma (POAG) is the most common type, a complex and heterogeneous disorder with changes in intraocular pressure (IOP) and some changes in metabolism contributing to the pathogenesis [1]. Glaucoma treatment is aimed at slowing the progression of glaucomatous optic neuropathy and stabilizing further visual field loss. The only effective treatment to slow primary open-angle glaucoma progression is the reduction of the IOP with anti-glaucomatous eye drops, laser or surgical treatments [2]. Surgical treatments may be necessary if pharmacological and/or laser treatments prove to be ineffective (i.e., target IOP is not achieved and/or there is further visual field loss) [3]. Glaucoma filtration procedures, although effective, are also known for their complications. This stimulates the development of minimally invasive glaucoma surgery (MIGS), because new approaches are required to combine a high hypotensive efficacy of glaucoma filtration procedures with a low rate of postoperative complications of MIGS. It is required to compare two surgical treatments, a gold standard (trabeculectomy) against non-penetrating deep sclerectomy combined with ab interno endotrabeculectomy, to determine which treatment is more appropriate in the particular clinical situation of each patient with POAG. The purpose of this study was to compare the efficacy of non-penetrating deep sclerectomy combined with ab interno endotrabeculectomy with that of trabeculectomy for primary open-angle glaucoma. Material and Methods Twenty-five patients (25 eyes) that received surgery for POAG were included in the study. They were divided into two groups based on the type of surgery received: trabeculectomy (13 patients; 13 eyes) and non-penetrating deep sclerectomy combined with ab interno endotrabeculectomy (12 patients; 12 eyes). There was no significant difference between groups with regard to age, gender and comorbidities. The mean age was 69.8 ± 8.35 years for patients in group 1, and 70.08 ± 6.93 for patients in group 2. Group 1 included 6 male patients and 7 female patients, and group 2 included 5 male patients and 7 female patients. Advanced (stage 3) glaucoma was diagnosed in 8 patients of group 1 and 8 patients of group 2, and terminal (stage 4) glaucoma, in 8 patients of group 1 and 4 patients of group 2. Gonioscopy was performed to verify the diagnosis. Trabecular pigmentation was mild, moderate and severe in 2 patients (2 eyes), 4 patients (4 eyes), and 7 patients (7 eyes), respectively, of group 1, and 4 patients (4 eyes), no patients, and 7 patients (7 eyes), respectively, of group 2. Patients underwent uncorrected and best-corrected visual acuity assessment, biomicroscopy of the anterior segment and fundus, and IOP readings were obtained with a Maklakoff tonometer. In addition, static perimetry was performed if visual acuity was sufficiently good. The number of hypotensive medications used before and after surgery was also noted. Follow-up duration was 12 months. Informed consent for surgical treatment was signed by all patients of the study In brief, trabeculectomy in patients of group 1 was conducted as follows. Epibulbar alcaine anesthesia 0.5% and parabulbar 2.0 lidocaine anesthesia 2.0 % was administered. A superior rectus fixation suture was placed. A 6-mm-long conjunctival incision was made posterior to the limbus. Thermocoagulation of the episcleral vessels was performed. The superficial scleral flap, trapezoid in shape, and approximately one-half scleral thickness, 5 mm at the limbus and 4 mm at the apex was outlined and dissected. Radial incision of the limbus was performed for identification of the Schlemm Canal. Sinusectomy, trabeculectomy, and basal iridectomy were performed. Sclera and conjunctiva were then closed with four and two, respectively, 10-0 interrupted sutures. Antibiotic (0.3 ml) and dexamethasone (0.5 ml) were injected beneath the conjunctiva. Finally, a dressing was applied to the eye. In brief, surgery in patients of group 2 was conducted as follows. Epibulbar alcaine anesthesia 0.5% and parabulbar 2.0 lidocaine anesthesia 2.0 % was administered. A superior rectus fixation suture was placed. A 6-mm-long conjunctival incision was made posterior to the limbus. Thermocoagulation of the episcleral vessels was performed. The superficial scleral flap, trapezoid in shape, and approximately one-third scleral thickness, 5 mm at the limbus and 4 mm at the apex was outlined and dissected. One-third thickness deep sclerectomy was performed within a 4 x 5 mm triangle (with the triangle base lying at the transparent portion of the cornea) to the margin of the Descemet membrane that involves the external wall of the Schlemm Canal. The juxtacanicular trabecular meshwork was removed with forceps. Sclera and conjunctiva were then closed with four and two, respectively, 10-0 interrupted sutures. A 1.2-mm anterior chamber paracenthesis was performed at 3 and 10 o’clock, lidocaine 1% was injected into the anterior chamber, and the anterior chamber was filled with viscoelastic. A goniolens was installed, and the inferior nasal trabecular meshwork was removed. The viscoelastic was washed out from the anterior chamber using an irrigation-aspiration system, and the paracentesis wounds were hydrated. Antibiotic (0.3 ml) and dexamethasone (0.5 ml) were injected beneath the conjunctiva. Finally, a dressing was applied to the eye. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Measures to ensure safety, health, rights and dignity of patients and to adhere to ethical standards were taken when conducting the study. Statistical analyses were conducted using Statistica 12.0 (StatSoft, Tulsa, OK, USA) software. The Wilcoxon t test and Mann-Whitney U test were used. Results Mean IOP values in group 1 and group 2 were 26.00 ± 5.21 mmHg and 25.75 ± 1.29 mmHg, respectively, preoperatively (with no significant difference between groups, р> 0.05), 16.00 ± 3.08 mmHg and 16.50 ± 1.68 mmHg, respectively, at day 7; 17.15 ± 1.46 mmHg and 17.75 ± 1.48 mmHg, respectively, at 1 month; 17.38 ± 1.33 mmHg and 17.42 ± 0.90 mmHg, respectively, at 3 months; 17.62 ± 1.04 mmHg and 17.08 ± 0.79 mmHg, respectively, at 6 months; and 17.69 ± 1.03 mmHg and 17.92 ± 0.67 mmHg, respectively, at 12 months (Table 1).
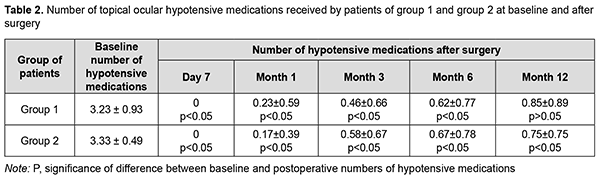
There was a significant difference between preoperative and any postoperative IOP levels at any time point (р< 0.05) for either group. In addition, there was no significant difference in IOP between groups (р> 0.05) at any time point. Mean numbers of ocular hypotensive medications used for group 1 and group 2 were 3.23 ± 0.93 and 3.33 ± 0.49, respectively, preoperatively (with no significant difference between groups, р> 0.05), 0 and 0, respectively, at day 7; 0.23 ± 0.59 and 0.17 ± 0.39, respectively, at month 1; 0.46 ± 0.66 and 0.58 ± 0.67, respectively, at month 3; 0.62 ± 0.77 and 0.67 ± 0.78, respectively, at month 6; and 0.85 ± 0.89 and 0.75 ± 0.75, respectively, at month 12 (Table 2).
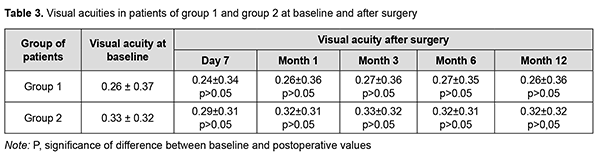
There was a significant difference between preoperative and any postoperative mean numbers of hypotensive medications used at any time point (р< 0.05) for either group. In addition, there was no significant difference in mean numbers of hypotensive medications used between groups (р> 0.05) at any time point. In group 1, of 13 patients, 7, 2 and 3 patients used 4, 3 and 2 hypotensive medications, respectively, preoperatively, and 3, 4 and 6 patients used 1, 2 and no hypotensive medications, respectively, at month 12. In group 2, of 12 patients, 4 and 8 patients used 4 and 3 hypotensive medications, respectively, preoperatively, and 5, 2 and 5 patients used 1, 2 and no hypotensive medications, respectively, at month 12. There was no significant difference between preoperative and postoperative visual acuity (р< 0.05) for either group (Table 3).
Our results demonstrate the efficacy of ab interno trabeculectomy combined with the removal of the juxtacanicular trabecular meshwork (ab externo) in patients with POAG. Our data demonstrate that the combined surgery is not less efficacious than penetrating glaucoma surgery. Mean percentage decrease in IOP from baseline at 6 months and 12 months was 32.23% and 31.96%, respectively, for patients of group 1, and 33.67% and 30.41%, respectively, for patients of group 2. Trabeculectomy as well as non-penetrating deep sclerectomy combined with ab interno endotrabeculectomy demonstrated an increase in the number of hypotensive medications used until month 12 after surgery. Of the patients of group 1, three exhibited ciliochoroidal detachment and hypotony in the early postoperative period. In two of the three patients, these complications did not require surgery, and the choroid was attached in all quadrants at days 7 and 10. A patient with a preoperative IOP of 38 mmHg had to receive the surgical drainage of the suprachoroidal space at day 5. It is noteworthy that the presence of this complication was not always dependent on the highest preoperative IOP in the group. Thus, in the three patients that developed ciliochoroidal detachment, a preoperative IOP was 22, 26 and 38 mmHg. Although choroidal detachment, impaired circulation in the retina and/or optic nerve, and hypotony are typical for glaucoma filtration procedures, none of these was observed in patients of group 2. In 3 patients of group 2, hyphema was observed early after surgery and completely resolved by day 7. Discussion Scant studies have compared the efficacy of removal of the trabecular meshwork ab interno combined with removal of the juxtacanicular trabecular meshwork ab externo, with that of trabeculectomy. A study by Sato and colleagues [4] aimed to investigate treatment outcomes in 360° suture trabeculotomy with deep sclerectomy combined with phacoemulsification and aspiration and intraocular lens implantation (360P-LOT + DS). Thirty-two eyes in 32 consecutive patients treated by 360P-LOT + DS for POAG with coexisting cataracts were retrospectively compared with 23 eyes in 23 consecutive patients treated by cataract surgery and 120° trabeculotomy with deep sclerectomy (120P-LOT + DS). Both groups showed a significant decrease in intraocular pressure starting at one month after surgery when compared with values before surgery. At 3, 6, 9, and 15 months after surgery, the intraocular pressure was significantly lower and the survival rate was significantly higher in the 360P-LOT + DS group compared with the 120P-LOT + DS group. The main idea of the modified surgical technique investigated in the current study was not in increasing the outflow through the site of removed juxtacanicular trabecular meshwork, but in (a) removing a large portion of the trabecular meshwork and providing access to the outflow tract and (b) improving aqueous circulation through the Schlemm Canal to the site of deep sclerectomy and removed juxtacanicular trabecular meshwork. In addition, unlike suture trabeculotomy, after trabecular meshowork removal, there will be no residual trabecular tissue capable of deteriorating outflow facility [5]. The main advantage of ab interno trabeculectomy and deep non-penetrating sclerectomy is a significantly reduced risk of hypotony, whereas the latter is common after penetrating trabeculectomy. Rabiolo and colleagues [6] aimed to report long-term outcomes of primary deep sclerectomy in POAG and identify factors influencing surgical failure and postoperative complications. They concluded that higher preoperative IOP was a predictor of increased failure. Therefore, it is important to seek opportunities to modify the available surgical techniques in order to improve their efficacy in disease treatment. Thus, in the current study, deep non-penetrating sclerectomy was combined with ab interno trabeculectomy. A study by Bendel and Patterson [7] aimed to evaluate the long-term safety and efficacy of ab-interno trabeculectomy with trabectome for the treatment of glaucoma. They found a statistically significant reduction in IOP (p < 0.01) at final follow-up (average = 18.35 months) of nearly 23%. In the current study, there was a reduction in IOP of 31.96% at 12 months after ab interno trabeculectomy combined with deep non-penetrating sclerectomy, indicating an advantage of the combined surgical procedure. Klemm [8] reviewed non-penetrating deep sclerectomy as an alternative to trabeculectomy. He noted that deep sclerectomy was developed to avoid the intraoperative and postoperative complications seen with trabeculectomy. The author concluded that there is a controversial discussion about the pressure lowering effect of deep sclerectomy in comparison to trabeculectomy. Some studies have shown a similar long-term efficacy for both procedures but others showed an advantage for trabeculectomy. The results of the current study demonstrated that ab interno endotrabeculectomy combined with non-penetrating deep sclerectomy has not less hypotensive effect than trabeculectomy. In addition, there was no significant difference in mean numbers of hypotensive medications used between groups treated with these techniques at any time point. Not only our combination glaucoma surgical procedure has at least the same hypotensive effect as that of trabeculectomy, but it is also advantageous in a low incidence of complications that are typical for glaucoma filtration procedures, which indicates that the procedure is both effective and safe. Conclusion First, endotrabeculectomy, when combined with non-penetrating deep sclerectomy, has a statistically significant hypotensive effect in POAG at any time point of the follow-up, which is not less than that of trabeculectomy. Second, there was a significant difference between preoperative and any postoperative mean numbers of topical IOP-lowering medications used at any time point for either group, and there was no significant difference between groups with regard to number of topical IOP-lowering medications used at 12 months after surgery. Finally, not only EDE combined with NPDS has at least the same ocular hypotensive effect as that of trabeculectomy for POAG, but it is also advantageous in a low incidence of severe postoperative complications, which may make it a procedure of choice for POAG.
Conflict of Interest Statement. The authors declare no conflict of interest. Funding Support. There are no external sources of funding.
References 1.Trivili A, Zervou M, Goulielmos GN, et al. Primary open angle glaucoma genetics: The common variants and their clinical associations (Review). Mod Med Rep. 2020 Aug;22(2):1103-10. 2.Sihota R, Angmo D, Ramaswamy D, Fafa T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018 Apr;66(4):495-505. 3.Bertaud S, Aragno V, Baudouin C, Labbé A. [Primary open-angle glaucoma]. Rev Med Interne. 2019 Jul;40(7):445-452. 4.Sato T, Hirata A, Mizoguchi T. [Outcomes of 360º suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open glaucoma]. Clin Ophthalmol. 2014 Jul 11;8:1301-10. 5.Novytskyy IIa. [Modern surgery of primary open-angle glaucoma. Transition to minimally invasive operations]. Lviv: Litopys; 2018: 106–119. Ukrainian. 6.Rabiolo A, Leadbetter D, Alaghband P, Anand N. Primary deep sclerectomy in open-angle glaucoma: long-term outcomes and risk factors for failure. Ophthalmol Glaucoma. 2021;4:149–61. 7.Bendel RE, Patterson M. Long-term Effectiveness of Trabectome (Ab-interno Trabeculectomy) Surgery. J Curr Glaucoma Pract. Sep-Dec 2018;12(3):119-124. 8.Klemm M. [Deep sclerectomy. An alternative to trabeculectomy]. Ophthalmologe. 2015 Apr;112(4):313–18. DOI: 10.1007/s00347-014-3161-6. German
|