J.ophthalmol.(Ukraine).2021;2:45-49.
|
http://doi.org/10.31288/oftalmolzh202234549 Received: 11.11.2020; Accepted: 02.06.2022: Published on-line 15.06.2022 Assessing the impact of anti-inflammatory interleukin IL-10 and flosteron, a long-acting corticosteroid, on the course of corneal burn process S. A. Iakymenko, O. V. Dzhygaliuk, M. V. Gavryliuk SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE:Iakymenko SA, Dzhygaliuk OV, Gavryliuk MV. Assessing the impact of anti-inflammatory interleukin IL-10 and flosteron, a long-acting corticosteroid, on the course of corneal burn process. J.ophthalmol.(Ukraine).2022;3:45-9. http://doi.org/10.31288/oftalmolzh202234549 Background: Ocular burns are the most severe injury to the eye, and their treatment is still a challenge. Purpose: To assess the temporal changes in and grades of major characteristics of the corneal burn process after anterior chamber injection of anti-inflammatory interleukin (IL)-10 versus subconjunctival injection of flosteron, a long-acting corticosteroid. Material and Methods: Eighteen Chinchilla rabbits were divided into 3 groups of six each, and the right eye of all rabbits received an experimental burn. Thereafter, the right eye of rabbits of group 1 received anterior chamber IL-10 injection, the right eye of rabbits of group 2, subconjunctival flosteron injection, and the right eye of rabbits of group 3 (the control group), a drop of Floxal in the conjunctival sac at days 3, 7, 21 and 30. Results: The main characteristics of corneal inflammatory process (corneal opacity (edema), ocular inflammation, corneal erosion, vascularization, and leucoma) changed with time after burn, and were closely associated with each other, but the scores of these characteristics were lower in the rabbits of groups 1 and 2 compared to controls. In addition, the scores were higher in the rabbits that received IL-10 injections compared to those that received subconjunctival flosteron injections. Conclusion: Subconjunctical corticosteroid injections can be reasonably used for inflammation in ocular burns, and are more advantageous than interleukin injections in the anterior chamber in terms of easy use, safety and availability. Keywords: ocular burns, IL-10, inflammatory process, corticosteroids, flosteron
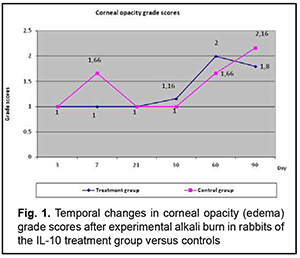
Introduction Ocular burns are the most severe injury to the eye. The annual incidence of ocular burns among adults in Ukraine has been reported to be 1.5-1.6 cases per 100,000 population [1], and their treatment is still a challenge. The burn process is complex and contains many components, and studies on the burn process are a major area aimed at the improvement of the efficacy of ocular burn treatment [2]. Studies on various components of the pathogenesis of ocular burn disease have been conducted at the Filatov institute over years, which enabled developing novel advanced treatment methods [2, 3, 4]. Inflammation develops in biological tissues as a response to any damage to the human body (e.g., burn damage), runs for certain time, through different phases, and has various clinical manifestations [5, 6]. When a burn occurs to the eye, a similar response develops in ocular tissues, which is evidenced by the symptoms of ocular inflammation (hyperemia and vascular injection, corneal and iris edema, anterior chamber exudation and ocular pain) that can be seen during examination of the eye or slit lamp examination [2, 3, 7]. However, the post-burn pathophysiological changes developing in ocular tissues and media have not been studied sufficiently yet. Cherikchi conducted biochemical analyses of eliminate (a substance obtained by passing direct current through the eye, with the anode being placed on the eye) to determine the amino acid composition of this substance [8]. Cherikchi [8], Iakymenko and colleagues [9], and Gladush [10] (1) examined the eliminate obtained by electric elimination in the affected eye of experimental animals and patients with ocular burns, and (2) demonstrated that ocular tissues of these subjects accumulated histamine, histidine, protein and amine nitrogen, and exhibited increased proteolytic activity, evidencing the presence of inflammation process in these tissues. In addition, the levels of histamine, histidine, and protein and amine nitrogen in the above tissues corresponded to the temporal changes in and severity of the ocular burn process. Studies on the role of interleukins in pathogenesis of various human diseases have been given much attention in recent years [11]. Others have reported on studies on the role of cytokines in ocular disorders like neovascular glaucoma, macular degeneration, uveitis, infectious and viral disorders. Consequently, we believe it is reasonable to study the role of anti-inflammatory interleukin (IL)-10 and flosteron, a corticosteroid drug, for better understanding of the pathophysiology of ocular inflammation in ocular burns. This is believed to be helpful in the diagnostic assessment and prediction of the course of ocular inflammation in ocular burns, and the development of advanced methods of treatment for the inflammation. The purpose of the study was to assess the temporal changes in major characteristics of the corneal burn process after anterior chamber injection of IL-10 versus subconjunctival injection of flosteron, a long-acting corticosteroid. Material and Methods Eighteen Chinchilla rabbits (weight, 2-2.5 kg) were used in the study and divided into 3 groups of six each. The right eye of rabbits of group 1 received a 0.3-ml anterior chamber IL-10 injection at days 3, 7, 21 and 30 after a 0.3-ml aqueous sample was obtained from the eye. The right eye of rabbits of group 2 received a 0.5-ml subconjunctival flosteron injection at days 3, 7, 21 and 30. A drop of ofloxacin (Floxal, Bausch & Lomb) was instilled in the conjunctival sac of the right eye of rabbits of the control group at days 3, 7, 21 and 30 after a 0.3-ml aqueous sample was obtained from the eye. Rabbits of the three groups were subjected to alkali corneal wounds in their right eyes. Following application of topical anesthetic (0.5% proparacaine hydrochloride; Alcaine, Alcon-Couvreur, Puurs, Belgium), a 10 mm diameter filter paper, soaked in NaOH solution (pH 13.6), was placed over the cornea in the right eye of each animal, and the cornea was exposed for 10 seconds to obtain a severe corneal burn (a corneal grade 3B burn) with the cornea having a ground glass appearance. The course of the corneal inflammatory burn process was documented by photography at the specified time points, and clinical characteristics were scored using the following scales: Corneal opacity (edema) (1, transparent cornea; 2, corneal clouding; 3, translucent opacity (with well seen pupil and iris contours); 4, severe opacity; 6, ground glass appearance); Corneal erosion size (calculated as half of the sum of vertical and horizontal diameters in millimeters); Corneal vascularization severity (0, no neovascularization; 1, individual vessels; 2, 3-5 vessels; 3, more than 5 vessels); Ocular inflammation severity (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation); and Corneal leucoma size (1, 5-6 mm; 2, 7-8 mm; 3, 9-10 mm). The corneal inflammation characteristics obtained after anterior chamber injection of IL-10 were compared with those of the control animals. In addition, the impact of anterior chamber injection of IL-10 on the rabbits of group 1 was compared with the impact of subconjunctival flosteron injection on the rabbits of group 2. Mean group scores were calculated for each characteristic. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Results First, we compared the temporal changes in characteristics of corneal inflammation between rabbits of group 1 (which received anterior chamber injections of IL-10) and control animals. Temporal changes in corneal opacity (edema) for rabbits of group 1 versus control animals Immediately after experimental burn injury, the cornea had ground glass appearance, and subsequent changes in corneal opacity (edema) in group 1 were close to those in controls (Fig. 1). Until day 21, corneal opacity (edema) in controls was somewhat more severe than in rabbits of group 1. After day 21, corneal opacity increased in both groups. At day 60, corneal opacity in rabbits of group 1 was somewhat more severe than in controls, but, after day 60, corneal opacity in the former rabbits was again similar to that in the latter rabbits. This is likely to indicate a negative impact of anterior chamber injection of IL-10 on corneal edema, possibly due to several corneal punctures.
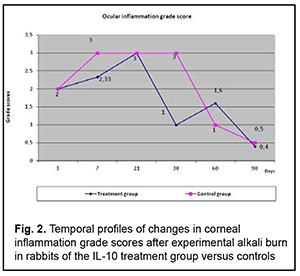
Grade of ocular inflammation The profile of temporal changes in ocular inflammation in rabbits of group 1 were almost similar to those in controls, with a peak score at day 7 and gradual decrease after day 21 (Fig. 2), but the grade of ocular inflammation in the former rabbits was lower than in the latter rabbits.
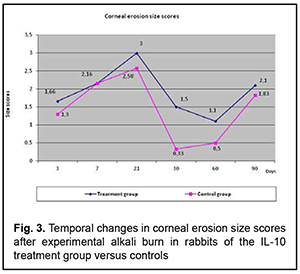
Temporal changes in the size of corneal erosion (Fig. 3)
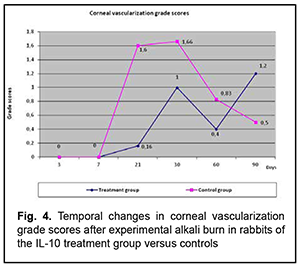
The profile of temporal changes in corneal erosion size scores in rabbits of group 1 was almost similar to that in controls, but corneal erosion diameter was larger the former rabbits until the end of the experiment. A possible cause of this fact is that frequent anterior chamber injections of IL-10 delayed corneal re-epithelialization. Temporal changes in and the grade of corneal vascularization (Fig. 4)
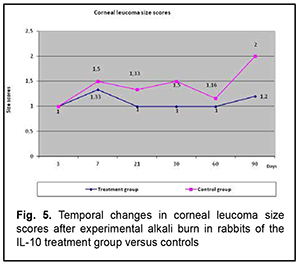
Vascular growth into the cornea began after day 7, increased until day 30, and occurred in parallel in both groups. However, the grade of corneal vascularization was significantly lower in rabbits of group 1 compared with controls. Temporal changes in the size and the grade of corneal opacity (leucoma) (Fig. 5)
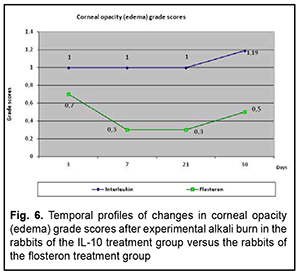
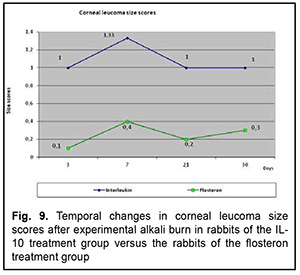
The profile of temporal changes in corneal leucoma size score in rabbits of group 1 was almost similar to that in controls, with an increase until day 7 followed by a decrease until day 60, although the size score was somewhat lower in rabbits of group 1 compared with controls. In the diagrams presented in the figures, the abscissa displays days after burn, and the ordinate, the score of the inflammatory process. Therefore, we compared the scores of the major characteristics of the corneal burn process between group 1 and controls, and found that the temporal changes in the scores of group 1 were closely associated with those of controls, but the scores were lower in the former rabbits than in the latter rabbits, which indicated a generally positive impact of anti-inflammatory interleukin of the corneal burn process. Then we compared the temporal changes in corneal inflammation characteristics between rabbits of group 1 which received anterior chamber injections of IL-10 and rabbits of group 2 which received subconjunctival flosteron injections. Temporal changes in corneal opacity (edema) The profile of temporal changes in corneal opacity (edema) for group 1 was similar to that for group 2 (Fig. 6). Immediately after experimental burn injury, the cornea had ground glass appearance, but the mean corneal opacity score was higher in the rabbits that received IL-10 injections compared to those that received subconjunctival flosteron injections at all time points.
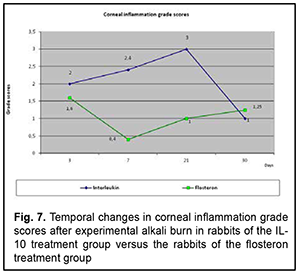
Grade of ocular inflammation The profile of temporal changes in ocular inflammation for group 1 was almost similar to that for group 2 (Fig. 7). However, the grade of ocular inflammation was higher in the IL-10 group than in the flosteron group until day 20, and it was only by day 30 that the grade of ocular inflammation in the former group became equal to that in the latter group.
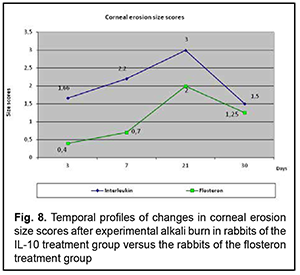
Temporal changes in corneal erosion size The profile of temporal changes in corneal erosion size for group 1 was almost similar to that for group 2 (Fig. 8). Corneal erosion size was larger in the IL-10 group than in the flosteron group until day 20, a decrease in erosion size in both groups began at day 20, and it was only by day 30 that the erosion size in the former group became equal to that in the latter group.
Temporal changes in corneal vascularization Vascular growth into the cornea began at day 7, and increased until day 30, but the grade of corneal vascularization was significantly higher in the IL-10 group than in the flosteron group. A decrease in the grade of corneal vascularization was observed from day 30 onwards in both groups. Temporal changes in and grade of leucoma size The profile of temporal changes in leucoma size for group 1 was almost similar to that for group 2 (Fig. 5). Leucoma size score increased until day 7 and gradually decreased thereafter, but leucoma size score was significantly larger in the IL-10 group than in the flosteron group.
Discussion In the rabbits that received anterior chamber injections of IL-10 after burn injury, the scores of corneal opacity (edema), ocular inflammation, corneal erosion size, vascularization, and leucoma size changed with time after burn in a manner similar to, but were somewhat higher than those in the rabbits that received subconjunctival flosteron injections. This demonstrated that subconjunctival corticosteroid administration is more advantageous for the treatment of ocular inflammation after ocular burn in terms of severity of injection-induced trauma, easiness and medication cost. In addition, our previous studies have demonstrated that subconjunctival corticosteroid injections exerted no effect on the temporal changes in ulceration, although no special studies have been conducted [3]. Therefore, our experimental studies demonstrated that the main characteristics of the burn process (corneal opacity (edema), ocular inflammation, corneal erosion, vascularization, and leucoma) changed with time after burn, and were closely associated with each other. The scores of corneal opacity (edema), ocular inflammation, corneal erosion size, vascularization, and leucoma size were lower in the rabbits that received anterior chamber injections of IL-10 after burn compared with control animals. It is likely that this difference was caused by the technique of administration of IL-10, periodic puncture of the cornea for injection of the medication. We compared the characteristics of burn process in the cornea between the rabbits that received anterior chamber injections of IL-10 and the rabbits that received subconjunctival flosteron injections after burn events, and found that the characteristics in the former rabbits were practically similar to those in the latter rabbits. This indicates that subconjunctical corticosteroid injections can be reasonably used for ocular burns, and are more advantageous than interleukin injections in the anterior chamber in terms of efficacy and severity of injection-induced trauma.
References 1.Gladush TI. [New opportunities provided by electrical elimination in studying, diagnosis and treatment of ocular burns]. Abstract of Cand Sc (Med) thesis. Odesa: Filatov Institute of Eye Diseases; 2004. Ukrainian. 2.Pasyechnikova NV, Rykov SO, Vitovska OP, Stepaniuk GI, Martoplias KV, Myrnenko VV. [Analyssis of the eye care provided to the population of Ukraine in 2006-2011]. Oftalmol Zh. 2012;6:131-40. Ukrainian 3.Puchkovskaia NA, Shulgina NS, Nepomiashchaia VM. [Involvement of autosensibilization in the development of late complications of ocular burns]. In: [Current issues of ophthalmology]. Kyiv; 1970. p.170. Russian. 4.Puchkovskaia NA, Iakimenko SA, Nepomiashchaia VM. [Eye burns]. Moscow: Meditsina; 2001. Russian. 5.Ronkina TI. [Morphological and histological changes in the anterior segment of the rabbit’s eye after local alkali injury to the cornea]. In: [Results of clinical and experimental studies. A collection of science works]. Moscow; 1973. p.181-3. Russian. 6.Serov VV, Paukov VS. [Inflammation]. Moscow: Meditsina; 1995. Russian. 7.Strukov AI, Saropsov DS, Serov VV, eds. [General human pathology: a guide for physicians]. Moscow: Meditsina; 1982. Russian. 8.Cherikchi LE. [Physiotherapy in ophthalmology]. Kyiv: Zdorov’ia; 1979. Ukrainian. 9.Chuklin SN, Pereiaslov AA. [Interleukins]. Lvov: Liga Aress; 2005. Russian. 10.Shul’gina NS. [Role of abnormality of immunobiological systems in the pathogenesis of the burn process in the cornea]. Oftalmol Zh. 1959;6:323. Russian. 11.Iakymenko SA, Kolomyichuk SG, Gladush TI. [Some biochemical characteristics in the ocular burn process]. Oftalmol Zh. 2002;3:5-10. Russian.
Disclosures Corresponding author. Dzhygaliuk O.V., email: dovgalenko1@ukr.net Author contribution. Iakymenko S.A.: design, participation in the experiment, data analysis, editing the article; Gavryliuk M.V.: conducting an experiment, initial data analysis, writing an article; Dzhiygalyiuk O.V.: participation in the experiment, data analysis, writing the article. All authors analyzed the results and approved the final version of the manuscript. Sources of support. None Conflict of interest. The authors do not have any real or potential conflicts of interest (financial, personal, professional and other interests) that could affect the content and conclusions of the work.
|