J.ophthalmol.(Ukraine).2022;5:3-11.
|
http://doi.org/10.31288/oftalmolzh20225311 Received: 07.07.2022; Accepted: 17.08.2022; Published on-line: 27.10.2022 Role of polymorphisms of folate-cycle enzymes in diabetic retinopathy progression in patients with type 2 diabetic mellitus S. O. Rykov 1, Iu. V. Prokopenko 1, L. V. Natrus 2, Iu. O. Panchenko 1 1 Shupyk National Healthcare University of Ukraine 2 Bogomolets national medical university Kyiv (Ukraine) TO CITE THIS ARTICLE: Rykov SO, Prokopenko IuV, Natrus LV, Panchenko IuO. Role of polymorphisms of folate-cycle enzymes in diabetic retinopathy progression in patients with type 2 diabetic mellitus. J.ophthalmol.(Ukraine).2022;5:3-11. http://doi.org/10.31288/oftalmolzh20225311
Background: Hyperhomocysteinemia is important in the development of endothelial dysfunction, a pathogenetic component of diabetic retinopathy (DR) in type 2 diabetes mellitus (DM2). The major cause of homocysteine accumulation is missing or dysfunctional enzymes and cofactors that are needed to perform homocystein metabolic processes. This is, for the most part, the deficiency of folate cycle enzymes determined by methylenetetrahydrofolate reductase (MTHFR) C677T, MTHFR A1298C, and methionine synthase (MTR) A2756G polymorphisms. Purpose: To assess the role of polymorphisms of folate cycle enzymes (MTHFR C677T, MTHFR A1298C, MTR A2756G) in DR progression in patients with DM2. Material and Methods: The study included 83 DM2 patients (83 eyes) in whom DR met the criteria of non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS). The control group was composed of 35 non-diabetics comparable to patients with respect to gender, age and body mass index. Real-time PCR was conducted on a Gene Amp® PCR System 7500 to determine polymorphisms. Plasma L-homocysteine levels were determined using an enzyme-linked immunosorbent assay (ELISA) microplate reader and Axis® Homocysteine Enzyme Immunoassay Kit (AXIS-SHIELD Diagnostics Ltd). Conclusion: We found no association of rs1801133, rs1805087, and rs1801131, folate-cycle polymorphisms, with the development of DR in DM2, compared with non-diabetics. In NPDR patients having the minor homozygous GG genotype of MTR 2756А/G and/or the minor homozygous CC genotype of MTHFR 1298A/C, plasma L-homocysteine levels were significantly increased. This requires further elucidation of the role of hyperhomocysteinemia as a factor of DR progression or as a marker of the severity of tissue injury. The duration of DM2 is an important factor of DR progression in carriers of the most common CC and CT genotypes of rs1801133, AG genotype of rs1805087, and АА genotype of rs1801131. Keywords: diabetic retinopathy, type 2 diabetes mellitus, folate cycle, homocystein, MTHFR C677T, MTHFR A1298C, MTRA 2756G polymorphisms
Introduction Diabetic retinopathy (DR) is still a major cause of loss of vision in working-age adults, so the problem is still relevant. Research is underway on all pathogenetic components of microvascular complications of type 2 diabetes mellitus (DM2), from systemic effects of nutritional factors, lifestyle, generic susceptibility, and impaired molecular mechanisms of lipid metabolism resulting in metabolic changes and leading to aggravation of DM2, to molecular mechanisms of biochemical effects of hyperglycemia, oxidative stress, impaired homeostasis, and changes in gene transcription associated with oxidative stress, increased endothelial apoptosis, and the role of mitochondrial dysfunction [1-3, 4]. However, in spite of advances in this field, the molecular mechanism of the multifactorial disease is still poorly understood [4, 5]. Experimental and clinical studies demonstrated that patients with DM2 and animals with experimental diabetes have increased levels of homocysteine both in blood and tissues [6-8]. An elevated homocysteine level is a dangerous factor and results in the development of endothelial dysfunction and microvascular complications (retinopathy, nephropathy, cardiomyopathy, and neuropathy), because high homocysteine causes structure damage to endothelial cells and disruption of the blood brain barrier, resulting in retinal ischemia and neovascularization, increased vascular endothelial growth factor (VEGF), and activation of endoplasmic reticulum stress and oxidative stress [9-12]. Homocysteine is a potential biomarker and a potential therapeutic target for DR [9]. Hyperhomocysteinemia is frequently associated with age and physiological features, and individual genetic, epigenetic and nutritional risk factors. The major cause of homocysteine accumulation is, however, missing or dysfunctional enzymes and cofactors (water-soluble vitamins B1, B2, and B3) associated with homocysteine metabolism, especially in the elderly [7, 8]. It is for this reason that the potential association of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C, and methionine synthase (MTR) A2756G polymorphisms of folate cycle enzymes with the risk for hyperhomocysteinemia has been studied. There have been reports describing nervous system abnormalities (including brain tissue inflammation) which result in the development of Alzheimer’s disease, Parkinson’s disease and child autism spectrum disorder, the disorders based on the genetically determined deficiency of folate cycle enzymes [13,14]. However, there is no agreement on whether there is an association between polymorphisms of the above genes and diabetic retinopathy in DM2. There have been reports on the association of MTHFR (677C/T) and DR in Asian populations [15-17]. There is also no agreement on whether these polymorphisms are associated with the development and progression of DM2 and its complications [8, 18, 19]. The purpose of the study was to assess the role of polymorphisms of folate cycle enzymes (MTHFR C677T, MTHFRA 1298C, MTRA 2756G) in DR progression in patients with DM2. Material and Methods Eighty-three DM2 patients with different stages of DR were included in the study. Stages of DR were determined using the early treatment diabetic retinopathy study (ETDRS) scale. This allowed dividing patients into two groups that were substantially different in terms of the severity of lesions, the group (43 patients, 43 eyes) including patients with mild, moderate or severe non-proliferative DR (NPDR), and the group (40 patients, 40 eyes) including patients with mild, moderate or severe proliferative DR (PDR). The control group was composed of 35 patients without DM2 who had no diagnosed metabolic abnormalities and presented for preventive examination to the Clinical and Diagnostic Laboratory of the university clinic of Bogomolets National Medical University. Approval for the study was obtained from the Bioethics Committee, the Shupik National Healthcare University of Ukraine. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association. Informed consent was obtained from all patients. Patients underwent an examination as per the ETDRS protocol including visual acuity assessment, refractometry, static Humphrey perimetry, tonometry, and biomicroscopy. In addition, gonioscopy, ophthalmoscopy with contactless aspheric lenses and a Goldmann contact lens, and macular optical coherence tomography (Topcon DRI OCT Triton, Tokyo, Japan) were performed, if required. Moreover, a fundus camera was used to examine the retina. Thyroid hormone levels were measured to exclude the presence of hormonal abnormality. Molecular and genetic studies were performed using standard techniques at the certified laboratories of the Bohomolets NMU Research Institute for Experimental and Clinical Medicine. Plasma L-homocysteine levels were determined using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Rt2100c; Rayto Life and Analytical Sciences Co. Ltd, Shenzhen, China), PST-60HL-4 microplate thermo-shaker (Biosan, Riga, Latvia), PW40 plate washer (Bio-Rad® Laboratories) and Axis® Homocysteine Enzyme Immunoassay (EIA) kit (AXIS-SHIELD Diagnostics Ltd, Dundee, Scotland, UK, Cat No. FHCY100). A venous blood sample was collected and transferred into a 4-ml K3 ethylenediaminetetraacetic acid (EDTA) blood collection tube in the laboratory procedure room. After centrifugation, the plasma was transferred to an Eppendorf tube for ELISA measurements, and the precipitated cell layer was stored at −20 °C until genetic analysis. Genomic DNA was extracted using PureLink® Genomic DNA Kits for purification of genomic DNA (Invitrogen Corp., Carlsbad, CA, USA). At the first phase, an aliquot of precipitated cells was incubated with proteinase K, RNase A and digestion buffer at 55°C for 10 minutes for cell lysis and RNA destruction. Centrifugation was used to perform DNA-column binding, purification and elution. The Taqman SNP Genotyping Assay (Applied Biosystems, Foster City, CA) and the ABI 7500 real-time PCR system (Applied Biosystems) were used to analyze polymorphic DNA loci. MTHFR C677T, MTHFR A1298C, and MTR A2756G polymorphisms were studied. Odds ratios (ORs), standard error of the odds ratio (S) and 95% confidence interval (CI) were calculated to determine the relative strength of the associations. The OR was calculated using the following formula:
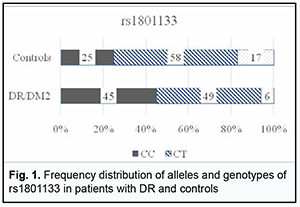
where A is the number of cases with a risk factor and an event of interest; B is the number of cases with a risk factor and no event of interest; C is the number of cases with an event of interest but no risk factor; D is the number of cases with neither a risk factor nor an event of interest. An odds ratio larger than 1 indicates a higher risk of the event in those exposed to the risk factor, and that the factor is directly associated with the event of interest. Therefore, the OR value indicates how much more likely one group is to have a particular problem than the group with which it is being compared. OR values were calculated using an on-line calculator (https://mathcracker.com/) and Microsoft Excel software. Data were analysed using IBM SPSS version 23 and MedStat software. Normality of quantitative data was established using the Shapiro–Wilk test. Most parameters were not normally distributed. Consequently, nonparametric statistical tests were used for statistical analyses at a significance level of 0.05. The descriptive statistical indices of median, and 25th and 75th percentile [Q1-Q3] for the groups are listed in tables. 95% confidence intervals for median and interquartile ranges were calculated. Bar charts are provided with 95% confidence intervals (CI). Kruskal-Wallis test was used for comparisons between groups. The Dunn’s test or the Mann-Whitney test with Bonferroni correction was used for pairwise comparisons. Normally distributed parameters are presented as means ± standard deviation. Results We analyzed allele and genotype frequency distributions for rs1801133, rs1805087 and rs1801131 in the groups of the study and calculated the relevant OR values to determine the role of folate cycle polymorphisms in DR in DM2. The data on rs1801133 are shown in Fig. 1 and Table 1.
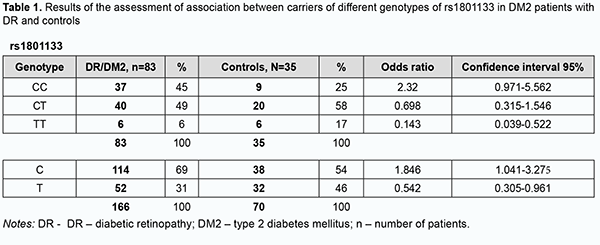
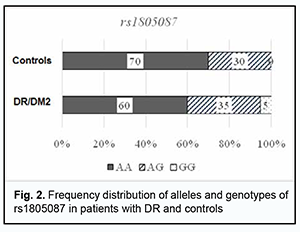
Among the controls, the heterogenous CT genotype was most common with 58%, followed by the CC (25%) and TT (17%). Among the patients, the heterogenous CT genotype was also most common, but the percentage was smaller than in the controls (49 versus 58%) at the expense of an increased percentage of the CC genotype (45%). In addition, the percentage of TT genotype was substantially smaller in patients compared with controls. Therefore, the rs1801133 СС genotype is more associated with the disease than the rs1801133 TT genotype. Odds ratios (ORs), standard error of the odds ratio (S) and 95% confidence interval (CI) were calculated to determine the relative strength of the associations (Table 1). OR values were 2.32 (95% CI, 0.971-5.562; P > 0.05) for the CC genotype, 0.698 (95% CI, 0.315-1.546; P > 0.05) for the CT, and 0.143 for the TT (95% CI, 0.039-0.522; P > 0.05). In patients, the C allele (69%) was two times more frequent than the T allele, whereas in controls, the former allele was just slightly more common than the latter allele, with OR values of 1.846 (95% CI,1.041-3.275; P > 0.05), and 0.542 (95% CI, 0.305-0.961; P > 0.05), respectively. Therefore, there was no evidence of association of rs1801133 with DR in DM2 compared to individuals without diabetes. Analysis of rs1805087 polymorphism association with DR showed (Fig. 2, Table 2) that, among the controls, the major AA genotype was more common than the heterogeneous AG genotype (70% versus 30%, respectively).
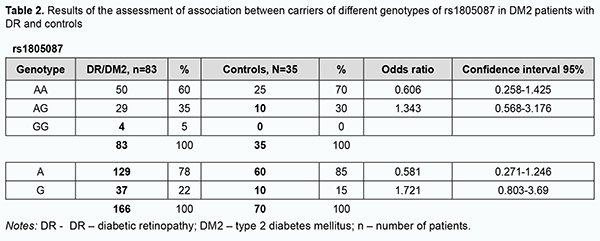
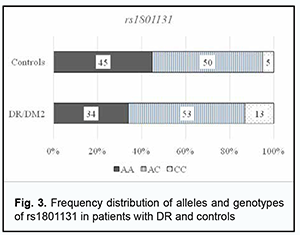
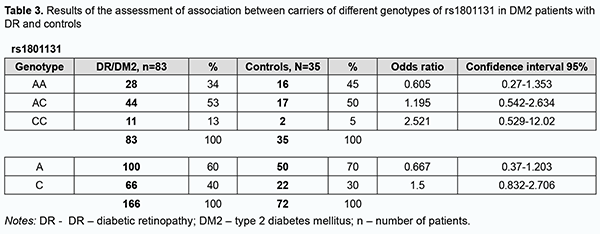
Among the patients, the AA genotype was also most common (60%), followed by the AG (35%) and GG (5%). In the comparison of polymorphism frequencies between patients and controls, OR values were 0.606 (95% CI, 0.258-1.425; P > 0.05) for the AA genotype and 1.343 (95% CI, 0.568-3.176; P > 0.05) for the AG. The A allele was most common among patients and controls, and almost 3.5-fold more common than the G allele in patients, and 5.6-fold more common than the G allele in controls. OR values were 0.581 (95% CI, 0.271-1.246; P > 0.05) for the A allele and 1.721 (95% CI, 0.803-3.69; P > 0.05) for the G allele. Therefore, there was no evidence of association of rs1805087 with DR in DM2. Analysis of rs1801131 polymorphism association with DR in DM2 showed (Fig. 3, Table 3) that, among the controls, the AC genotype was most common (50%), followed by AA (45%) and CC (5%) genotypes. The pattern of distribution of these genotypes among patients was similar to that in controls, with the AC genotype being most common (53%), followed by AA (34%) and CC (13%) genotypes. OR values were 0.605 (95% CI, 0.27-1.353; P > 0.05) for the AA genotype, 1.195 (95% CI, 0.542-2.634; P > 0.05) for the AC, and 2.521 for the CC (95% CI, 0.529-12.02; P > 0.05). The A allele was most common among patients and controls, it was 2.3-fold more common than the C allele in patients, and 1.5-fold more common than the C allele in controls. OR values were 0.667 (95% CI, 0.37-1.203; P > 0.05) for the A allele and 1.5 (95% CI, 0.832-2.706; P > 0.05) for the C allele.
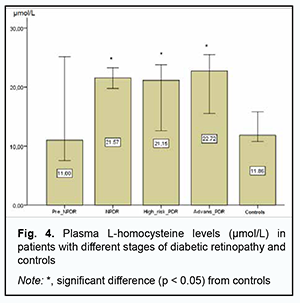
Therefore, there was no evidence of association of rs1805087, rs1805087, and rs1801131, important polymorphisms of folate cycle enzymes, with DR in DM2. Given potential effects of high plasma L-homocysteine levels on the development of vascular dysfunctions and properties and state of the endothelium, particularly in the presence of hyperglycemia, we assessed median [interquartile] levels of plasma L-homocysteine in DR patients with DM2 and controls. We found that median [interquartile] levels of plasma L-homocysteine were twice higher in patients compared to controls, 21.12 µmol/l (15.41-24.57) versus 11.85 µmol/l (10.63-16.96), respectively, and this difference was significant (р < 0.05). In addition, there was only a slight difference in median [interquartile] levels of plasma L-homocysteine between patients with NPDR and those with PDR, 20.86 µmol/l (15.09-25.15 versus 21.68 µmol/l (16.02-23.76). We subdivided the NPDR group into the Pre_NPDR (n=12) and moderate NPDR (n=21) subgroups, and the PDR group into the High_risk_PDR (n=20) and Advanc_NPDR (n=20) subgroups and compared the subgroups for median [interquartile] levels of plasma L-homocysteine. We found plasma L-homocysteine levels to increase already at the onset of NPDR, with median [interquartile] (Min-Max) levels of plasma L-homocysteine to be 11.0 µmol/l [9.11÷19.27] (7.56-25.15) for the Pre_NPDR subgroup and 11.85 µmol/l [10.6÷16.9] (6.57-25.89) for the controls, which reflects a substantial variation among patients. An increase in the severity from pre-NPDR to moderate NPDR was accompanied by an almost twofold increase in plasma L-homocysteine levels.
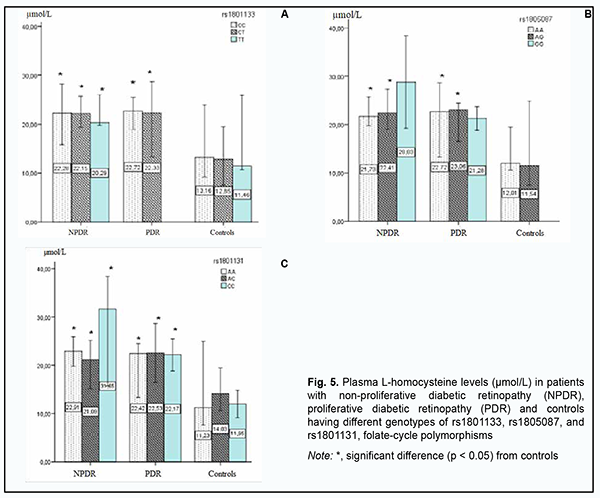
Therefore, although we found no association of the genetically determined deficiency of folate cycle enzymes with the risk of developing DR in DM2, plasma L-homocysteine level may be considered a marker of the development of microvascular DM2 complications, particularly, DR. Consequently, we believe it is important to keep searching for potential trigger factors for structural retinal damage and increased severity of retinopathy in patients with DM2. Fig. 5 shows the results of the analysis of plasma L-homocysteine level in DM2 patients with NDR or PDR depending on rs1801133, rs1805087, and rs1801131, genetically determined polymorphisms. Across all patient carriers of SNP rs1801133, there was a twofold increase in plasma L-homocysteine level (р < 0.05), with no substantial change in plasma L-homocysteine level with an increase in the severity of retinal damage. For carriers of AA or AG, the most common genotypes of rs1805087АА, plasma L-homocysteine levels were twofold higher in patients with NPDR or PDR compared to controls (р < 0.05), with no significant difference between groups of patients. However, it is interesting that, in NPDR patients having the minor GG genotype of rs1805087, plasma L-homocysteine levels were 1.3-fold higher than in NPDR patients having another genotype than GG, and 2.4-fold higher than in controls, and there was no significant difference in plasma L-homocysteine level between PDR patients having the GG genotype and PDR patients having another genotype than GG.
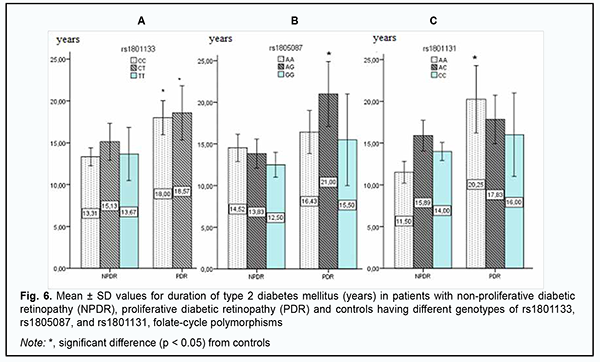
Given that there were no carriers of GG genotype among controls, we may hypothesize that this genotype is a potential trigger factor for structural retinal damage and increased severity of retinopathy in patients with DM2. A similar pattern was observed among carriers of rs1801131, with plasma L-homocysteine levels being twofold greater in carriers of AA or AC, the most common genotypes of rs1801131, compared to controls. In addition, in NPDR patients having the minor CC genotype of rs1801131, plasma L-homocysteine levels were threefold higher than in controls, and 1.4-fold higher than in NPDR patients with another genotype than CC. High plasma L-homocysteine levels in NPDR patients having the GG genotype of rs1805087 and/or the CC genotype of rs1801131 may be discussed as a potential mechanism of initiation of retinal damage, but the current study was limited by a small number of carriers of these genotypes, and this point requires further research. For further search of triggers of DR progression in DM2, groups of patients and variants of the genetically determined deficiency of folate cycle enzymes were compared for DM2 duration (Fig. 6). The mean diabetes duration was 13.6 ± 6.51 years for the NPDR group and 16.8 ± 6.47 years for the PDR group, with no significant difference between the groups. Since there was no significant difference between the NPDR and PDR groups with regard to mean DM2 duration or DR progression in the current study, we should search for other potential mechanisms that could predispose a patient to an increase in the severity of retinal damage.
Therefore, a more detailed analysis of the relationship between DM2 duration and genetically determined folate cycle deficiency demonstrated that, in carriers of the most common CC and CT genotypes (and, probably, the C allele) of rs1801133, type 2 diabetes duration is an important factor of DR progression, because there was a significant difference in diabetes duration between patients with NPDR and those with PDR. In addition, DM2 duration exerted an effect on DR progression in patients with the AG genotype of rs1805087 and patients with the AA genotype of rs1801131, showing a statistically significant 1.5-fold (р < 0.05) and 1.7-fold (р < 0.05) increase, respectively, in DM2 duration and DR progression with an increase in DM2 duration in NPDR and PDR groups. Moreover, there was a tendency for an increase in retinopathy severity with an increase in DM2 duration in carriers of other genotypes of the SNPs (rs1801133, rs1805087, and rs1801131) that determine folate cycle deficiency, but this factor was not a major one in the pathogenesis of the progression of this complication. Discussion Therefore, our analysis of the distribution of genotypes and alleles of rs1801133, rs1805087, and rs1801131, in our groups of DM2 patients with DR demonstrated that, in a genetic variant in MTHFR (677C/T, rs1801133), the heterozygous genotype CT is a major genotype found in 58% of the controls, which is likely to be a factor preventing the progression of DM2, because we found that (a) the development of DR was accompanied by a decrease in the percentage of CT carriers at the expense of an increased percentage of CC genotype carriers, and (b) the percentage of carriers of the latter genotype among patients was 1.8-fold higher than among controls. In addition, we demonstrated that, in carriers of the most common CC and CT genotypes (and, probably, the C allele) of rs1801133, type 2 diabetes duration is an important factor of DR progression. A study by Raza and colleagues [18] evaluated MTHFR gene polymorphisms in 175 North Indian subjects including 87 DM2 cases and 88 controls. The authors did not find an association between MTHFR gene and DM2 cases, because the MTHFR gene CC, CT, TT genotype frequencies obtained were 40%, 43% and 17% in DM2 cases and 56%, 29% and 15% in healthy controls, respectively. In addition, The OR for CC was 0.54 (95%CI, 0.29-0.98; P = 0.041), for CT, 1.76 (95% CI 0.94-3.30; P = 0.07), and for TT, 1.2 (95% CI, 0.53-2.70; P = 0.66). It seemed to them that MTHFR gene CC genotype is a good marker for early identification of population at risk of DM2, but further study with larger groups was required to validate that study [18]. Niu and Qi [15] conducted a meta-analysis of studies investigating the association of MTHFR gene 677C/T polymorphism with DR, with a total of 1599 subjects from 8 studies analyzed for DR. Carriers of 677TT genotype were 1.86 times (95% CI, 1.21-2.86; P = 0.004) more likely to develop diabetic retinopathy separately relative to diabetic patients without DR and non-diabetic controls. Subgroup analysis showed that ethnicity was a possible confounder, especially in West Asians and Africans [15]. Xu and colleagues [16] were of a similar opinion. In the current study, the percentage of carriers of the TT genotype was 2.8-fold higher among controls compared to patients, with low probability of developing DR in them. We, however, noted that the current study is limited by a low number of carriers of potential DR-associated genotypes. Our analysis of genotypes of MTR 2756А/G (rs1805087) demonstrated that the major homozygous genotype AA was seen in 70% of controls and 60% of patients. In addition, the duration of DM2 was a leading factor of DR progression in carriers of the AG genotype. Moreover, the homozygous GG genotype was found in patients, but not in controls, and thus the presence of this genotype may be considered a risk factor for DR. In NPDR patients having this genotype of rs1805087, plasma L-homocysteine levels were 1.3-fold higher than in NPDR patients having another genotype than GG, and 2.4-fold higher than in controls, and this difference was statistically significant. Our analysis of genotypes of MTHFR 1298A/C (rs1801131) demonstrated that half of study patients had the heterozygous AC genotype which was not associated with the development of the disease. The homozygous AA genotype was also not associated with the development of the disease, and the duration of DM2 was an important factor of DR progression in carriers of this genotype. In addition, the minor CC genotype was 2.6-fold more common in patients than in controls. Moreover, in NPDR patients having this genotype of rs1801131, plasma L-homocysteine levels were threefold higher than in controls, and 1.4-fold higher than in NPDR patients with another genotype than CC. Conclusion First, we found no association of rs1801133, rs1805087, and rs1801131, folate-cycle polymorphisms, with the development of DR in DM2, compared with non-diabetics. Second, in NPDR patients having the minor homozygous GG genotype of MTR 2756А/G and/or the minor homozygous CC genotype of MTHFR 1298A/C, plasma L-homocysteine levels were significantly increased. This requires further elucidation of the role of hyperhomocysteinemia as a factor of DR progression or as a marker of the severity of tissue injury.
Finally, the duration of DM2 is an important factor of DR progression in carriers of the most common CC and CT genotypes of rs1801133, AG genotype of rs1805087, and АА genotype of rs1801131. References 1.Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2017 Dec 1;102(12):4343-4410. 2.Shi C, Wang P, Airen S, et al. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis (Lond). 2020 Jun 18;7:33. 3.Natrus LV. A novel concept of differences in pathogenetic mechanism of diabetic retinopathy progression between type 2 diabetes mellitus patients differing in the PPARγ genotype. J Ophthalmol (Ukraine). 2020;5:36-42. 4.Kowluru RA. Diabetic Retinopathy: Mitochondria Caught in a Muddle of Homocysteine. J Clin Med. 2020 Sep 19;9(9):3019. 5.Kowluru, RA, Mohammad G, &Sahajpal N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis (Lond). 2020 Jan 10;7:4. 6.Malaguarnera G, Gagliano C, Giordano M, et al. Homocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathy. Biomed Res Int. 2014;2014:191497. 7.Koklesova L, Mazurakova A, Samec M, et al. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021 Nov 11;12(4):477-505. 8.Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients. 2021 Dec 20;13(12):4562. 9.Tawfik A, Mohamed R, Elsherbiny N, et al. Homocysteine: A Potential Biomarker for Diabetic Retinopathy. J Clin Med. 2019 Jan; 8(1):121. 10.Mogіlevskyy SIu, Panchenko IuO, Zyablіtsev SV. Predicting the risk of diabetic retinopathy-associated macular edema in patients with type 2 diabetes mellitus. J Ophthalmol (Ukraine). 2019;3:3-8. 11.Mogіlevskyy SIu, Panchenko IuO, Zyablіtsev SV. Value of TNF-α (rs1800629) polymorphism in recurrent maculopathy after surgery in a Ukrainian population of T2DM patients. J Ophthalmol (Ukraine). 2019;6:15-22. 12.Panchenko IuO. [Relationship of PDGFB rs1800818 polymorphism with recurrent diabetic maculopathy after surgical treatment in patients with type 2 diabetes mellitus]. Vіsnyk problem bіologії і medytsyny. 2019;4-1(153): 138-42. Ukrainian. 13.Maltsev D, Natrus L. The Effectiveness of Infliximab in Autism Spectrum Disorders Associated with Folate Cycle Genetic Deficiency. Psychiatry, psychotherapy and clinical psychology. 2020; 11(3):581-92. Russian. 14.Maltsev D. Features of folate cycle disorders in children with ASD. Bangladesh Journal of Medical Science. 2020; 19(4):737–42. 15.Niu W, Qi Y. An updated meta-analysis of methylenetetrahydrofolate reductase gene 677C/T polymorphism with diabetic nephropathy and diabetic retinopathy. Diabetes Res Clin Pract. 2012, 95(1):110-8. 16.Xu WH, Zhuang Y, Han X, Yuan ZL. Methylenetetrahydrofolate reductase C677T polymorphism and diabetic retinopathy risk: a meta-analysis of the Chinese population. J Int Med Res. 2020;48(1):300060518816834. 17.Luo S, Wang F, Shi C, Wu Z. A Meta-Analysis of Association between Methylenetetrahydrofolate Reductase Gene (MTHFR) 677C/T Polymorphism and Diabetic Retinopathy. Int J Environ Res Public Health. 2016; 13(8):806. 18.Raza ST, Abbas S, Ahmed F, Fatima J, Zaidi ZH, Mahdi F. Association of MTHFR and PPARγ2 gene polymorphisms in relation to type 2 diabetes mellitus cases among north Indian population. Gene. 2012, 15;511(2):375-9. 19.Zhong JH, Rodríguez AC, Yang NN, Li LQ. Methylenetetrahydrofolate reductase gene polymorphism and risk of type 2 diabetes mellitus. PLoS One. 2013 Sep 4;8(9):e74521.
Disclosures Corresponding Author: Larysa Natrus, e-mail: lnatrus777@gmail.com Authour Contribution: All authors meet the criteria of authorship, certify that each author has substantially contributed to the work, including conception, design, analysis, writing and revision of the article, and each author is responsible for its content. Sources of Support: There are no external sources of funding. Conflict of Interest Statement: All authors have no real or potential conflicts of interest (financial, personal, professional, or other) that could affect the subject matter or material described and discussed in this manuscript. Sources of support: There are no external sources of funding.
Study Participants: All participants gave informed consent to participate in the study. The study was conducted in compliance with the requirements of the Declaration of Helsinki, the Council of Europe Convention on Human Rights and Biomedicine (1977), the relevant provisions of the WHO, the International Council of Medical Scientific Societies, the International Code of Medical Ethics (1983) and the Order of the Ministry of Health of Ukraine № 690 of 23.09.2009.
|