J.ophthalmol.(Ukraine).2022;6:44-49.
|
http://doi.org/10.31288/oftalmolzh202264449 Received: 14.09.2022; Accepted: 05.10.2022; Published on-line: 21.12.2022 Optic Neuritis or Inflammatory Optic Neuropathy: a review N. M. Moyseyenko Ivano-Frankivsk National Medical University; Ivano-Frankivsk (Ukraine) TO CITE THIS ARTICLE: Moyseyenko NM. Optic Neuritis or Inflammatory Optic Neuropathy: a review. J.ophthalmol.(Ukraine).2022;6:44-49. http://doi.org/10.31288/oftalmolzh202264449 According to the literature, there are two equivalent terms "optic neuritis" and "inflammatory optic neuropathy". Classification, pathogenesis and clinical manifestations are ambiguous. However, the growing interest in the problems of demyelinating and infectious diseases contributes to detailing the data on the mechanisms of certain forms of optic nerve inflammation. Purpose. To study the current views on the pathogenesis of optic nerve inflammation. Methods. The literature search in Ukrainian and foreign scientific sources was performed. Modern diagnostic methods such as optical coherence tomography (OCT), magnetic resonance imaging (MRI), immunological and virological tests allow studying i) the role of factors, individual and in combinations, in the development of neuritis, ii) the changes in visual functions, iii) damage to individual structural elements of the visual system, and iv) recovery from the damage received. Conclusion. The analysis of literature data on optic neuritis and/or inflammatory optic neuropathy showed a high topical interest of the scientific community. Further studies are likely to improve the current classifications of optic nerve inflammation and develop a differentiated approach to the treatment of optic nerve inflammation. Keywords: optic neuritis, inflammatory optic neuropathy, OCT, MRI, demyelinating diseases
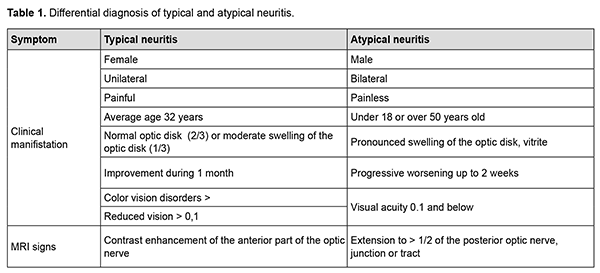
Optic neuritis [1] and inflammatory optic neuropathy [2] are two terms which can be found in the literature and which explain a large group of inflammations of the optic nerve. The discussion on the correct use of the terms and the understanding of the nature of damage requires more detailed research and literature search. Purpose. To study the current views on the pathogenesis of optic nerve inflammation. Methods. The literature search in Ukrainian and foreign scientific sources (32 sources). The classification of neuritis, theories of pathogenesis and approaches to their treatment in Ukrainian ophthalmology and in the world societies of neuro-ophthalmology (NANOS and EUNOS) are different. In Ukraine, the most commonly used classification is based on localization (papillitis and retrobulbar neuritis) [3], while abroad they prefer the classification based on pathogenesis (typical and atypical neuritis). Until recently, up to 2016, the difference between typical neuritis, which is considered a consequence of demyelinating diseases, and atypical neuritis, which has a different nature, was clearer (Table 1). [4].
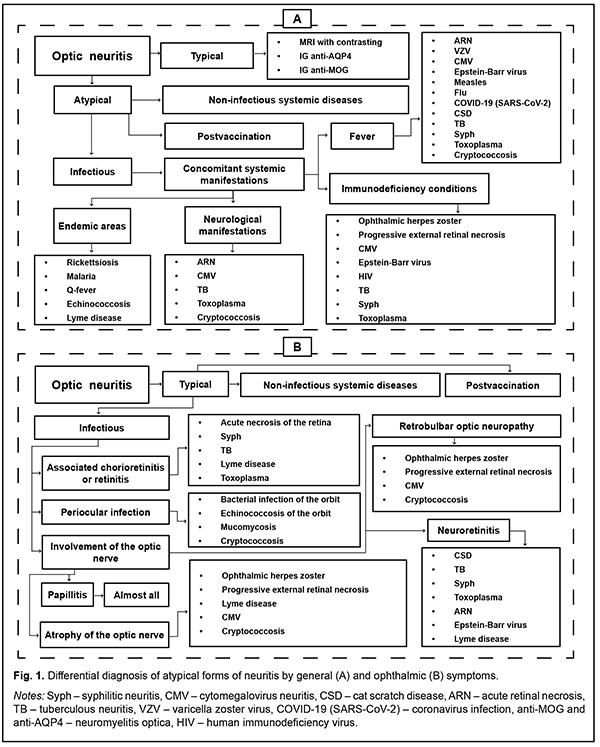
On the other hand [5], the diversity of manifestations of atypical forms, in which both papillitis and retrobulbar neuritis variants are possible (Fig. 1), shows, in our opinion, the imperfection of the Ukrainian neuritis classification system.
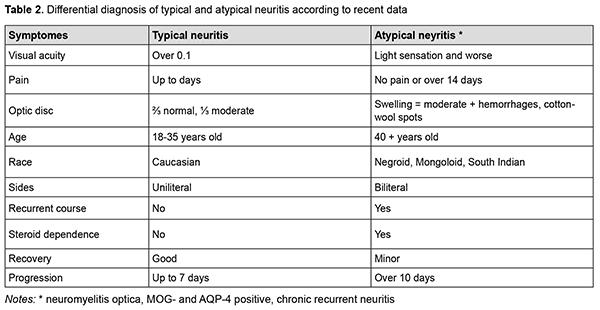
Now, a more thorough study of multiple sclerosis and its early manifestations has shown the heterogeneity in the group of demyelinating diseases. The occurring optic neuritis also has a different course (recurrent or monophasic), different visual functional outcomes (with full recovery or with the development of atrophy) and different treatment approaches (use of corticosteroids, monoclonal antibodies or plasmapheresis). An important cause of optic neuritis is a type of demyelinating disease, neuromyelitis optica (NMO) or Devic disease [6], which is characterized by unilateral neuritis, brainstem, cerebral and diencephalic syndromes. [7]. NMO is often associated with positive tests for antibodies to oligodendrocyte glycoprotein (MOG) and/or aquaporin-4 (AQP4-IgG) [8]. Due to modern research, the difference between typical and atypical neuritis (Table 2) is less pronounced, but this interpretation becomes the key to successful treatment, since it prevents the transformation of multiple sclerosis into more aggressive forms. [9].
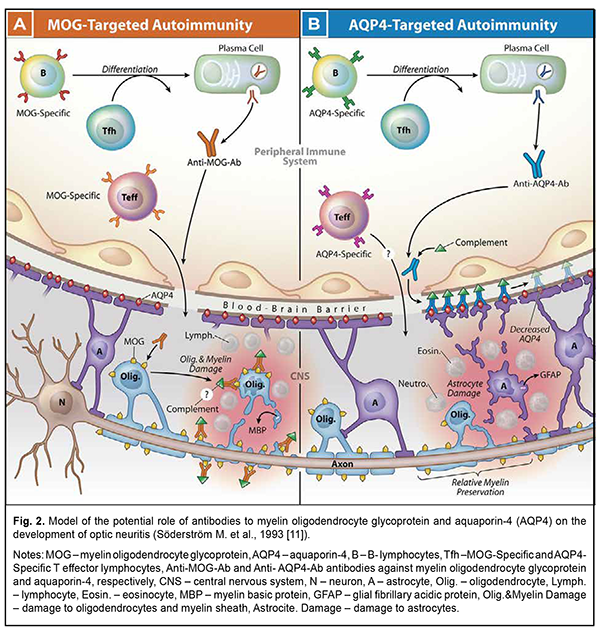
Pathogenesis of nervous tissue inflammation or inflammatory neuropathy development Systemic activation of T cells is observed at the onset of symptoms and precedes changes in the cerebrospinal fluid, which is considered the basis of demyelinating processes. [10]. In typical optic neuritis, there is also activation of B cells against the myelin basic protein in the cerebrospinal fluid. [11]. MOG-specific and AQP4-specific antibodies (Ab) target two different populations of resident cells of the central nervous system (CNS), oligodendrocytes (Olig.) or astrocytes (A), respectively. The data indicate that antibodies are produced outside of the CNS in both MOG Ig1 AQP4-seronegative opticospinal inflammatory disease (Fig. 2, A) and AQP4-seropositive neuromyelitis optica spectrum disorder (NMOSD) (Fig. 2 B). AQP4-specific antibodies interact with antigen-specific follicular T helper (Tfh) cells when B cells differentiate into plasma cells. [12].
Serum antibodies to MOG or AQP4 separately are not considered pathogenic in the absence of a cell-mediated inflammatory response. MOG-specific T effector cells (Teff) (which are detected in experimental autoimmune encephalitis (EAE), in multiple sclerosis (MS) [13]) or AQP4-specific Teff cells can initiate CNS inflammation, which in AQP4-seropositive NMOSD is characterized by the accumulation of neutrophils (Neutro.) and eosinophils (Eosin.). In both cases, inflammation can compromise the integrity of the blood-brain barrier, allowing the penetration of antibodies. MOG-specific antibodies are likely to bind MOG expressed on myelin-forming oligodendrocytes and the myelin layer surrounding axons which extend from the neuronal body (N) [14]. The pathogenesis of atypical, infectious forms of neuritis is much less studied. However, inflammation of the optic nerve against the background of activation of herpesvirus [15] and cytomegalovirus infection [16] is increasingly common. Immunological and virological studies of cerebrospinal fluid become mandatory in the diagnosis of neuritis forms [17]. In particular, the detection of antibodies [18] and oligoclonal plates [19] is the current standard for the diagnosis of multiple sclerosis, as it is many years ahead of the appearance of MRI foci [20]. Virological tests of cerebrospinal fluid can diagnose rare neuritis [21, 22]. Hemodynamic disorders in neuritis are studied by determining the thickness of the peripapillary choroid using optical coherence tomography (OCT) and magnetic resonance imaging (MRI). It was found that edema observed in papillitis leads to cerebral hypoperfusion in multiple sclerosis and optic neuromyelitis [23]. Some MRI studies indicate a decrease in perfusion and ischemia. Ischemia itself is classified as primary, which is associated with inflammation, and secondary, which occurs as a result of hypoperfusion after axonal degeneration [24]. On the other hand, hypoperfusion potentiates mitochondrial energy deficiency and oxidative stress, which also leads to axonal degeneration [25]. Impaired blood circulation in the extraocular vessels in multiple sclerosis has also been described. It leads to an increase in the concentration of endothelin-1 in the blood, which activates astrocytes around the disc head in optic papillitis and impairs surrounding perfusion [26]. It is also believed that hypoperfusion of the choroid is positively correlated with the degree of edema of the nerve fiber layer and their compression due to edema of the anterior part of the optic nerve [27]. Under the influence of ischemia, erythropoietin is also activated, which, in turn, activates signaling cascades. These cascades increase brain resistance to ischemia-reperfusion stress by stabilizing mitochondrial membranes and, thus, limit the formation of active intermediate oxygen and nitrogen compounds. The production of pro-inflammatory cytokines and neutrophil infiltration is suppressed. Therefore, if vascular hypoperfusion is part of the pathophysiology of acute optic neuritis, then erythropoietin may provide neuroprotection [28]. OCT in inflammatory neuropathy. This question is raised in case of chronic inflammatory processes such as neuromyelitis optica or multiple sclerosis. It is reported that in recurrent neuritis there is an increase in the thickness of the nerve fiber layer and a decrease in the thickness of the ganglion cell layer [29]. In MOG-associated acute neuritis, the nerve fiber layer is thinner compared to that in acute neuritis with multiple sclerosis [30, 31]. In acute neuritis, there is also an increase in the thickness of the outer and inner nuclear layers of the retina. Although some authors also report a decrease in the thickness of the ganglion cell layer in acute neuritis [32]. It is observed that the thickness of the complex of ganglion cells and the inner reticular layer reaches its maximum 4 months after acute neuritis, and then decreases; however, it remains thickened for 12 months compared to the baseline [33]. Thus, according to the literature, there are two equivalent terms - "optic neuritis" and "inflammatory optic neuropathy". Classification, pathogenesis and clinical manifestations are ambiguous. However, the increased interest in the problems of demyelinating diseases contributes to detailing the data on the mechanisms of certain inflammatory forms of the optic nerve. Modern diagnostic techniques, such as OCT, MRI, immunological and virological tests allow to study i) the role of individual factors, as well as their combination in the development of neuritis, ii) the changes in visual functions, iii) damage to individual structural elements of the visual system, and iv) recovery from the damage received. To conclude, analysis of the literature data on optic neuritis and/or inflammatory optic neuropathy showed a high current interest of the scientific community. Further advanced research is likely to improve the current classifications of optic nerve inflammation and develop a differentiated approach to the treatment of optic nerve inflammation.
References 1.Günay Ç, Yardım E, Yaşar E, Hız-Kurul AS, Uzan GS, Öztürk T et al. Optic neuritis in CD59 deficiency: an extremely rare presentation. Turk J Pediatr. 2022; 64(4): 787-94. 2.Eslami M, Lichtman-Mikol S, Razmjou, S, Bernitsas E. Optical Coherence Tomography in Chronic Relapsing Inflammatory Optic Neuropathy, Neuromyelitis Optica and Multiple Sclerosis: A Comparative Study. Brain Sci. 2022; 12: 1140. 3.Protocol of medical care for patients with optic neuritis (papillitis, retrobulbar neuritis). Annex to the Order of the Ministry of Health №117 dated 15-03-2007. 4.Critical review: Typical and atypical optic neuritis. Anne Abel, Collin McClelland, Michael S. Lee. J. Survophthal. 2019. 64 (6). P. 1-10. 5.Liang, J., et al. Comparing evolvement of visual field defect in neuromyelitis optica spectrum disorder-optic neuritis and idiopathic optic neuritis: a prospective study. BMC Ophthalmol 22, 338 (2022) 6.Kitchens N., Nichols L., Hope T. Educational Case: Neuromyelitis optica. Acad Pathol. 2022 Aug 16;9(1):100041. 7.Kahloun R, Abroug N, Ksiaa I, Mahmoud A, Zeghidi H. et al. Infectious optic neuropathies: a clinical update. Eye Brain. 2015 Sep 28; 7: 59-81. 8.Trebst C., Jarius S., Berthele A, et al. Update on the diagnosisand treatment of neuromyelitis optica: Recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. 2014; 261(1): 1e16. 9.Vanda A. Lennon. at al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. The Lancet. 2004; 364 (9451): 2106-2112. 10.Roed H, Frederiksen J, Langkilde A, Sørensen TL, Lauritzen M, Sellebjerg F. Systemic T-cell activation in acute clinically isolated optic neuritis. J. Neuroimmunol. 2005; 162(1-2): 165. 11.Söderström M, Link H, Xu Z, Fredriksson S. Optic neuritis and multiple sclerosis: anti-MBP and anti-MBP peptide antibody-secreting cells are accumulated in CSF. Neurology. 1993; 43(6): 1215. 12.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev. Immunol. 2011; 29: 621–663. 13.Shetty A. et al. Immunodominant T-cell epitopes of MOG reside in its transmembrane and cytoplasmic domains in EAE. Neurol. Neuroimmunol. Neuroinflamm. 2014; 1: e22. 14.Francis PJ, Jackson H, Stanford MR, Graham EM. Inflammatory optic neuropathy as the presenting feature of herpes simplex acute retinal necrosis. Br J Ophthalmol. 2003 Apr; 87(4): 512-4. 15.Melancia D, Fernandes A, Manita M, Cordeiro IM. Cytomegalovirus optic neuropathy in a young immunocompetent patient. J. Neurovirol. 2021 Apr; 27(2): 364-366. 16.Sarkar P, Mehtani A, Gandhi HC, Dubey V, Tembhurde PM, Gupta MK. Atypical optic neuritis: An overview. Indian J. Ophthalmol. 2021; 69(1): 27-35. 17.Rolak LA, Beck RW, Paty DW, Tourtellotte WW, Whitaker JN, Rudick RA. Cerebrospinal fluid in acute optic neuritis: experience of the optic neuritis treatment trial. Neurology. 1996 Feb; 46(2): 368-72. 18.Skov AG, Skov T, Frederiksen JL. Oligoclonal bands predict multiple sclerosis after optic neuritis: a literature survey. Mult Scler. 2011 Apr; 17(4): 404-10. 19.Olesen MN, Soelberg K, Debrabant B, Nilsson AC, Lillevang ST, Grauslund J et al. Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: a population-based prospective cohort study. J Neuroinflammation. 2019 Mar 11; 16(1): 59. 20.Rodríguez-Rodríguez MS, Romero-Castro RM, Alvarado-de la Barrera C, González-Cannata MG, García-Morales AK, Ávila-Ríos S. Optic neuritis following SARS-CoV-2 infection. J. Neurovirol. 2021; 27(2): 359-363. 21.Kahloun R, Abroug N, Ksiaa I, Mahmoud A, Zeghidi H, Zaouali S. et al. Infectious optic neuropathies: a clinical update. Eye Brain. 2015; 7: 59-81. 22.Varrin-Doyer M. et al. MOG transmembrane and cytoplasmic domains contain highly stimulatory T-cell epitopes in MS. Neurol Neuroimmunol Neuroinflamm. 2014; 1: e20. 23.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. Feb 2015; 2(1): e62. 24.D’Haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. Vascular aspects of multiple sclerosis. The Lancet Neurology. 2011; 10(7): 657-666. 25.Saindane AM, Law M, Ge Y, Johnson G, Babb JS, Grossman RI. Correlation of diffusion tensor and dynamic perfusion MR imaging metrics in normal-appearing corpus callosum: support for primary hypoperfusion in multiple sclerosis. AJNR Am Journal of Neuroradiology. 2007; 28(4): 767–772. 26.Cambron M, D’Haeseleer M, Laureys G, Clinckers R, Debruyne J, De Keyser J. White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. Journal of cerebral blood flow and metabolism. 2012; 32(3): 413-424. 27.Pache M. et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. European neurology. 2003; 49(3): 164-168. 28.Chen T-C. et al. Vascular hypoperfusion in acute optic neuritis is a potentially new neurovascular model for demyelinating diseases. PLoS ONE. 2017; 12(9): e0184927. 29.Nguyen AQ, Cherry BH, Scott GF, Ryou MG, Mallet RT. Erythropoietin: powerful protection of ischemic and post-ischemic brain. Experimental biology and medicine (Maywood, NJ). 2014; 239(11):1461-1475. 30.Eslami M, Lichtman-Mikol S, Razmjou S, Bernitsas E. Optical Coherence Tomography in Chronic Relapsing Inflammatory Optic Neuropathy, Neuromyelitis Optica and Multiple Sclerosis: A Comparative Study. Brain Sci. 2022; 12: 1140. 31.Chen JJ, Sotirchos ES, Henderson AD, Vasileiou ES, Flanagan EP, Bhatti MT. et al. OCT retinal nerve fiber layer thickness differentiates acute optic neuritis from MOG antibody-associated disease and Multiple Sclerosis: RNFL thickening in acute optic neuritis from MOGAD vs. MS. Mult. Scler. Relat. Disord. 2022; 58: 103525. 32.Kaushik M, Wang CY, Barnett MH, Garrick R, Parratt J, Graham SL. et al. Inner nuclear layer thickening is inversely proportional to retinal ganglion cell loss in optic neuritis. PLoS ONE. 2013; 8: e78341. 33.Al-Louzi OA et al. Outer retinal changes following acute optic neuritis. Mult. Scler. 2016; 22: 362–372.
Disclosures Disclaimer: The opinions expressed in this article are my own and not the official positions of the institution. Conflict of Interest Statement: The author has no actual or potential conflicts of interest (financial, personal, professional and other interests) that could influence the opinion on the subject matter or materials presented and discussed in this manuscript. Sources of Support: There are no sources of support.
|