J.ophthalmol.(Ukraine).2015;5:58-67.
|
https://doi.org/10.31288/oftalmolzh201555867 Immune Mechanisms, Clinical Features and Course of Non-infectious Uveitis M.V. Sydorova,1 Cand. Sc. (Med), V.Ie. Kondratiuk,2 Dr. Sc. (Med), Prof., N.G. Bychkova,2 Dr. Sc. (Med), Prof. 1 LLC Medical Center Dobrobut-Poliklinika, 2 Bogomolets National Medical University, Kyiv, Ukraine Е-mail: mariasydorova@gmail.com In most countries, the uveitides are responsible for 5-13% of all cases of blindness. Non-infectious uveitides are divided into those with and without known systemic association (both in children and adults). The Standardization of Uveitis Nomenclature (SUN) Working Group used anatomic location of the inflammation as an ontologic dimension, defined the criteria for the activity of inflammatory process, divided uveitis into groups and subgroups based on its course, and defined the tactical treatment algorithm. The uveitis development is based mainly on the alterations in the mechanisms of immunotolerance toward self ocular antigens, and on the emergence of autoagression toward these antigens. An alteration in immune tolerance to ocular antigens occurs if (1) regulatory CD25 + T cell subset is insufficient, and (2) B cells are activated with production of specific antibodies to the uveal tract structures. Systemic therapeutical agents for uveitis include corticosteroids, nonsteroidal anti-inflammatory drugs, cytostatics and immunobiologic agents. There are, however, no clinical guidelines involving the uveitis treatment approaches listed. The analysis of correlations between the degree of the alterations in the immune system and the course of uveitis will allow the development of the algorithm for the treatment of uveal inflammation entities. Keywords: uveitis, rheumatic diseases, the immune system, clinic, treatment
Introduction Since the uveitides are highly prevalent, tend to make restoration of visual functions difficult to evolve, and, in 76 % of cases, follow a recurrent course, studying them is an issue that must be urgently addressed [1, 2, 3]. Uveitis ranks as the fourth most common blinding disease in most countries, behind macular degeneration, diabetic retinopathy and glaucoma, and is responsible for 5-13% of all cases of blindness [4, 5, 6]. It has an annual incidence of 12 to 15 per 100,000 in Ukraine [7], with a worldwide prevalence ranging from 36 to 40 per 100,000 adults [8; 9; 10]. The disease is acute, recurrent, or chronic in one third, 55-59%, and 4-6%, respectively, of uveitic patients. These forms, although different in clinical signs and course, are common in the basic pathogenesis involving alterations in immune tolerance to ocular antigens [7, 11, 12]. Uveitis results in temporary or permanent incapacitation and reduced visual acuity, thereby significantly influencing the quality of life. The main age group affected is 25-55 years. Although men and women are approximately equally affected, idiopathic uveitis is more common in women, whereas systemic disease associated uveitis is found mostly in men. Classification of Uveitis In 2005, the Standardization of Uveitis Nomenclature (SUN) Working Group (WG) presented the classification which divides uveitis into anterior uveitis (iritis, iridocyclitis and anterior cyclitis), intermediate uveitis (pars planitis, posterior cyclitis and hyalitis), posterior uveitis (chorioretinitis and neuroretinitis), and panuveitis [13]. Table 1 shows the anatomic uveitis classification as per the SUN WG. In 2008, the International Uveitis Study Group (IUSG) designed an etiology-based classification of uveitis (infectious, non-infectious and that presenting as masquerade syndrome) [14]. Non-infectious uveitides are divided into those with and without known systemic association (both in children and adults). The former include idiopathic uveitis, Fuchs’ syndrome, glaucomatocyclitic crisis, phacolytic uveitis, toxic uveitis, allergic uveitis, posttraumatic uveitis, and postoperative uveitis [3, 14], whereas the latter include rheumatic-associated (human leukocyte antigen (HLA) B-27-associated) uveitis, Beh?et's disease-associated (HLA B-5 and B51-associated) uveitis, and juvenile rheumatoid arthritis (JRA)-associated (HLA DR5-associated) uveitis [3, 14, 15].
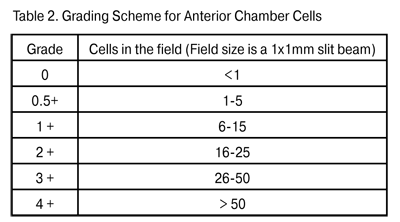
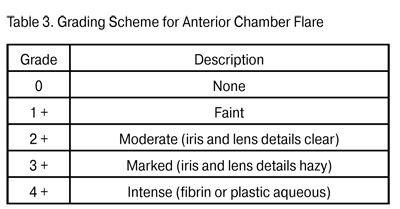
In 1973, HLA-27 antigen was found to have the most significant HLA haplotype association with systemic rheumatic diseases. The protein encoded by HLA-27 is used in presentation of various antigens (including their self cell determinants) to effector T lymphocytes [12, 16]. As a result, there emerges a subset of active T cells which then migrate to the target tissue to start the inflammatory process; it can involve several organs or systems simultaneously and lead to arthritis, dermatitis and uveitis. Elimination of autoagressive T cells ensures immune tolerance to self-tissues [17, 18, 19]. Uveitides can be classified according to the course as acute, recurrent and chronic. Acute anterior uveitis is idiopathic (in 50% of cases), or may be associated with seronegative spondyloartritis, Beh?et's disease or Reiter's syndrome. The term “recurrent uveitis” means that between attacks there is a period of inactivity without treatment of at least three months. The mean duration of an attack of acute uveitis or of a repeated episode is about two weeks, with the maximum up to four weeks. In about a half of uveitis patients, repeated episodes occur 2 to 3 times a year; however, such episodes have been reported to occur as late as 5 to 10 years after the initial episode [4, 20]. Recurrent uveitis is mostly HLA B-27-associated; the number of repeated episodes and disease activity in such cases correlate with the systemic inflammatory activity of [21, 22]. A torpid course of recurrent HLA-B27-positive uveitis eventually changing to the chronic form, although rare, has been reported, and mostly related to patients with enteropathy [3, 15, 20]. Recurrent uveitis without systemic and HLA association should be meticulously assayed for viral infection. Recurrent uveitis and HLA-B27 antigen are important signs to raise clinical suspicion of association with a systemic disease. In uveitis associated with a systemic disease, repeated episodes occur 2 to 3 times a year, whereas the frequency and activity of uveitis correlate with joint syndrome scores, C-reactive protein level, erythrocyte sedimentation rate, and serum rheumatoid factor. The most common systemic uveitis syndromes are seronegative spondylarthritis (Bekhterev's disease), reactive arthritis, rheumatoid arthritis, enteropathic arthritis and juvenile rheumatoid arthritis (JRA) [1, 5, 23]. Anterior uveitis occurs in 25-30% of patients with Bekhterev's disease, whereas conjunctivitis and recurrent uveitis occur in almost all (96%) and 11%, respectively, of those with Reiter's syndrome [5, 15, 24]. Anterior uveitis is found in 7-9% of patients with psoriatic arthritis [18, 25], whereas bilateral chronic uveitis (sometimes with asymmetric choroiditis) is found in 5-7% of patients with nonspecific ulcerative colitis [3, 4, 5]. JRA is the most common systemic disease associated with pediatric uveitis, accounting for 30% of all diagnoses, with as much as 3-4 recurrent episodes a year. In JRA children, the mean age at uveitis onset is 5.9 years, and that at arthritis onset is 4.8 years [5, 15, 23]. Chronic uveitis is found in 4-6% of uveitis patients; it is caused by persistent inflammation with relapse within three months after discontinuation of the treatment, and requires antiinflammaroty management of acute attacks [2, 22]. Fuchs’ syndrome, glaucomatocyclitic crisis, and JRA-associated uveitis are the most common chronic uveitis diseases. Mechanisms of immunotolerance toward ocular antigens Uveitis pathogenesis is intimately associated with the immune reactions which occur when the mechanisms of immunotolerance toward self ocular antigens break down and autoagression toward these antigens emerges [12, 26, 27]. The current opinion is that, in inflammatory diseases, the character of immune response depends on prevailing activation of specific T cell subsets which can migrate to uveal tract, synthesize various cytokines and trigger a local inflammatory response, with T and B cells playing a primary role [17, 28, 19]. Notwithstanding the presence of immunocompetent cells in all ocular tissues, normally, the immune response does not develop due to numerous immunotolerance mechanisms provided by nature both intraocularly and in immune supervisors. The specificity of intraocular immune processes is associated with the absence of lymphatic vessels in the eye, a specific structure of the capillaries in the iridociliary zone and choroid, and the choroid acting as a repository of lymphoid elements [3, 29]. Macrophages, dendritic cells and T cells of the iris and choroid have been identified as self antigen or foreign antigen presenting cells (APC) [18, 26]. The ocular APCs, in contrast to those from other organs and systems, do not migrate to the regional lymph node, but travel with the blood to the thymus and spleen [18, 26] where there is a system to control and suppress the macrophages and lymphocytes which are autoreactive towards self ocular antigens [19, 30]. According to numerous investigators, immunotolerance toward ocular antigens is ensured, above all, by blood retinal barrier and blood-uveal barrier protection of ocular antigens from the immune system: in the early ontogenesis, T memory cells are formed in immune supervisors to destroy autoreactive cell subsets. Additionally, the ocular APCs have no major histocompatibility complex (MHC) class II molecules (only class I proteins are phylogenetically present) required for activation of T and B cells in the thymus and spleen [21; 28]. If however this protection fails and autoreactive T cells are formed and migrate to the interstitial space of the uveal tract, regulatory CD25+FOXP3+ T cells found in the intercellular spaces of the uveal tract can suppress a local delayed type hypersensitivity response [31, 32]. An additional local factor for suppressing the autoreactivity toward ocular antigens is the production of complement blocking factors, immunosuppressive factor TGF-?2, ?-melatonin stimulating hormone (?-MSH), vasoactive intestinal peptide (VIP) and cortisol-binding globulin by fibroblasts, gliocytes and macrophages of the uveal tract [17, 27]. If even this defense mechanism proves to be ineffective, there is another one provided by nature: fibroblasts and macrophages of the iridal and ciliary stroma bear the apoptosis receptor Fas, also termed CD95; binding of these cells to activated T and B cells results in apoptosis of lymphocytes. Moreover, retinal gliocytes and macrophages bear the apoptosis receptor CD200R+, which is specific for the eye; binding of this receptor to autoreactive T cells results in suppressing the production of proinflammatory cytokines IL-1?, IL-6, and TNF-?, thereby stopping their interaction with ocular antigens [6]. All the prerequisites mentioned are involved into an ocular immunotolerance mechanism. In 1981, Strelein et al. were the first to describe this phenomenon and termed it Anterior Chamber Immune Deviation (ACAID). Strelein has paid special attention to the cell processes in the iris and anterior chamber of the eye, and proved that the anterior and posterior uveal tracts differ significantly in the mechanisms employed to develop the immune response. The ocular APCs (macrophages and dendritic cells) bearing receptor F4/80+ and influenced by the immune suppressive factors (including TGF-?2) become capable of inducing ACAID. It means that they capture the antigen in the intercellular spaces or ocular chambers via endocytosis, and deliver it to the spleen and thymus. There the antigen is recognized, memory T lymphocytes are developed, and ocular antigen delivering cells are destroyed by specific cytotoxic T cells. That is, normally, there is no production of the antibodies against the eye-derived antigens [19; 33]. In 1995, Streilein identified the active and passive factors that promote immune tolerance of eye-derived antigens. The active factors involve the following: natural expression of apoptosis-inducing molecules (FasL) and inhibitors of complement (CD59, MCP, DAF) in the retina and choroid, and immunosuppressive ocular tissue microenvironment containing TGF-?, VIP, ?-MSH etc. [19]. These factors cause apoptosis and immune-mediated cell lysis of autoagressive T cells which get into the uvea and lead to inflammation. The passive immune tolerance factors involve the following: the blood-ocular barrier, the absence of lymphatic drainage routes, and ocular cell expression of major histocompatibility complex (MHC) class I molecules only, which inhibits APC presentation of eye-derived antigens to T cells. The capacity for ocular and lymph node immune tolerance is however not always adequate to inhibit autoreactive T cell proliferation. The inherited susceptibility to autoimmune processes, when combined with antigenic mimicry between bacterial/viral endotoxins and synovium/uveal tract proteins, results in impairment of immune tolerance and production of antibodies against self-antigens [3, 8, 26]. Experimental uveitis studies In 1910, Elschnig [34] was the first to develop a concept of an uveitogenic antigen in the retina. Later Wacker [35] used animal models to show that uveitis can be induced after intraocular injection of retinal emulsion. The following studies of initiation of uveitis were based on the use of purified retinal S-antigen (55 kDa). In different animals, this antigen was introduced parenterally into different body sites distant from the eye. As a result, an acute uveal inflammatory processes differing in level of activity emerged in all the animals [36]. Hankey et al. described and used another retinal antigen, interphotoreceptor retinoid binding protein (IRBP) (140 kDa), to induce uveitis experimentally. They found that that the inflammatory response induced by this protein had less apparent exudative manifestations and was more chronic than that induced by retinal S-antigen [37]. The more recent history of uveitis animal studies involves different models of parenteral immunization with the following ocular antigens with Freund’s adjuvant: soluble retinal antigen (S-Ag), IRBP and melanin-associated antigen. Although the forms of uveitis differed in clinical features, in 96% of cases, the immunization-induced uveitis was acute, with no repeated episodes [32, 36, 37]. Shao et al. were the first to develop a successful animal model of recurrent uveitis [38]. Uveitogenic T cells were prepared from the draining lymph nodes after active immunization with R16 peptide R16, and introduced into the vitreous of na?ve rats. Over the following 80 days the recipient rats developed acute uveitis with several recurrences. The clinical score and duration of recurrent episodes depended on the number and activation status of T cells transferred, with these numbers and status regulated by the duration of T cell blast culturing in IL-2-containing medium prior to the injection. Thus, injection of a low (3 х 106) number of active T cells caused the early (3-4 days post injection) onset of active uveitis with frequent recurrences. Injection of a lower (0.5 х 106) number of active T cells caused the delayed (6-7 days post injection) onset of acute and monophasic uveitis with a low clinical score. The authors explain this as follows: active T cells find their way to the uveal tract and initiate inflammation; however, because the number of T cells is low, they are successfully eliminated and blocked by local defense mechanisms and cause no recurrent inflammation. There was no principal difference in histology between the acute monophasic uveitis and relapsing process: monocytic and lymphocytic infiltration was found in the presence of interstitial edema [38]. Immune homeostasis studies have been performed also with other activated T cell subsets, regulatory CD4+CD25+FoxP3-Т cells in particular: their numbers were found to be reduced significantly in blood, lymphatic nodes, ocular tissue and joints of patients with systemic diseases and animal models [28, 31, 39]. These cells, when taken from the animals with the acute monophasic uveitis and introduced parenterally to those with recurrent uveitis, resulted in elimination of recurrences, i.e., the resolution of uveitis [31, 32]. The authors believe that such an effect of CD4+CD25+FoxP3-Т cells is associated with their suppression of other activated T cells and produces IL10 and TGF-?2, both of which are capable of inhibiting the local inflammatory process in the uveal tract. Therefore, although some arms of the immunologic disorder and autoantibody production impairment in uveitis have been revealed, numerous issues related to the autoimmune processes common to the eye, joints, skin and mucosae are still unsolved, and there is some uncertainty related to the site of primary damage to immune tolerance. Additionally, the question is still open regarding the influence of the ocular- antigen autoreactive T cells on synovium, joint ligament, skin and mucosal structures. Answering this question requires analysis of both proinflammatory and inhibiting substances and the presence of specific T cell subsets in patients with acute and recurrent uveitis and different levels of inflammatory activity in the eye and joints. Correlation analysis of clinical and immunological data will allow identification of clinical and immunological risk factors for relapse of uveitis and the transition from acute to chronic uveal inflammation. Clinical characteristics of uveitis Anterior uveitis is usually unilateral; however, it is alternatively bilateral, albeit asymmetric, in 13-19% of cases, and simultaneously bilateral in up to 5% of cases [15, 20]. The clinical symptoms of uveitis correspond to the location of inflammation in the eye [3, 4, 22]. Thus, in anterior uveitis, patients usually complain of pain, photophobia, redness, blurred vision, and there is biomicroscopic evidence of cell detritus in the anterior chamber, iridal edema or spasm of the iridal sphincter muscle. Precipitates and fibrin deposit on the lens can result in alterations in aqueous humor dynamics with the development of complicated uveal glaucoma. Pain syndrome and injection of the globe are characteristic for all anterior uveitis entities because of (1) the blood supply type and (2) innervation of the iris and ciliary body by trigeminal fibers. The only exception to this principle is JRA-associated anterior uveitis: the inflammation is asymptomatic and painless, which makes it difficult to identify the disease correctly and provide effective treatment [3, 10, 16]. An example of success of the SUN WG was the development of inflammation grading schemas for anterior chamber cells (Table 2) and anterior chamber flare (Table 3). Each recurrence causes cell re-migration to the anterior chamber, and exudation of cytokines, growth factors, and fibrinogen to the anterior aqueous humor and tissues. All these things together result in alterations in aqueous humor dynamics, opacification of the optical media, and interstitial retinal edema, which significantly decreases the chances for complete visual recovery [8, 16, 23]. Nevertheless, under some circumstances, ophthalmologists miss some of the signs of inflammation during the routine examination of the anterior chamber. Fundus and peripheral retina examination should be perfomed with wide-angle contact lenses, and standard fundus photos should be used for grading vitreous cells [3, 15]. Acute anterior uveitis can be the only early clinical manifestation of a systemic disease, even with no pain in the lumbosacral spine and peripheral joints: in about one-fourth of patients with acute НLА-В27-associated uveitis, the initial examination reveals a systemic disease of connective tissue [12, 16, 17]. A typical unilateral sudden onset uveitis with pain and photophobia is a manifestation of a systemic connective tissue disease with high clinical and laboratory activity scores.
A feature of the course of rheumatic disease-associated uveitis is the involvement of inflammation of the uveal tract and other ocular structures (the conjunctiva, retina, sclera etc.), which significantly worsens the prognosis [4, 10, 22]. Additionally, according to Drozdova [4], in 37% and 22% of rheumatoid and psoriatic arthritis patients, the involvement of both scleral inflammation and uveitis has resulted in the development of necrotizing scleritis and peripheral corneal ulcers, respectively. In patients with a systemic disease, generalized uveitis is diagnosed less often (3-5%) than other forms [3, 5, 20]. Among the posterior uveitis entities which have been described are diffuse chorioretinitis and focal chorioretinitis with macular edema and edema of the healthy optic nerve [3, 4, 7]. Evidence has been reported of the development of isolated posterior uveitis in Reiter's disease presenting as focal central or peripheral chorioretinitis with the development of multiple small or solitary large foci and exudation under the retina, resulting in retinal detachment [10]. In enteropathic patients, ocular manifestations can present as serous retinal detachment resulting from diffuse choriocapillaris vasculitis [3]. JRA-associated uveitis has the following features: asymptomatic onset, chronic painless iris and ciliary body inflammation, with early clinical manifestation of limbal widening, retinal opacification at the lower limbus, and vitreous destruction and liquefaction [23]. In the later course, retinal crescent shaped opacities will appear at the palpebral fissure, at the 2-4 and 8-10 o’clock positions. The disease is characterized by the classic triad of ocular abnormalities (uveitis, band keratopathy, and complicated cataract) [15, 20]. JRA-associated uveitis may be accompanied by the inflammation of the healthy optic nerve which follows the pattern of papillitis and retrobulbar neuritis [10, 17]. There is, however, a category of patients with systemic connective tissue diseases who do not develop uveitis, despite high activity of the disease. This paradox might be explained by the genetic features of the HLA complex and high activity of immunosuppressive substances and immunoregulatory T cell subsets in the eye, lymph nodes and thymus. Uveal tract inflammation certainly influences intraocular structures, and one of the first complications of anterior uveitis is uveitic cataract that is associated with alterations in the content of the aqueous and inflammatory detritus and fibrin deposit on the lens surface. The most increased lens opacification rate is found in chronic recurrent uveitis, and the increase in cataractogenesis might depend on whether glucocorticoids are administered as intravitreal implants and systemic therapy [3, 40, 41]. Alterations in choriocapillaris and retinal vascular circulation occur in uveitis entities having different locations. In anterior uveitis, there are dystrophic iris and sclerotic trabecular meshwork changes. Cystoid macular edema (CME) is found in 8-19% and every third of anterior uveitis and choroiditis patients, respectively. According to Uy et al. [16], CME occurs in 13.4% of HLA-B27-positive uveitis patients, and the prognosis for the development of retinal edema can be made based on the number of vitreous inflammatory cells. CME development is caused by alterations in blood-ocular barrier and retinal vessels, and intraretinal accumulation of exudates (in the retinal outer plexiform layer and inner nuclear layer), which results in significant loss of vision. Nussenblatt affirms that CME is certainly accompanied by the edema of a healthy optic nerve, which may be subclinical and not diagnosed during routine ocular examination: neuropathy will be manifested only as enlargement of the blind spot and reduced contrast sensitivity [3]. In the presence of ocular hypotension and on the transition of acute to chronic inflammation, CME is completed with steady macular alterations, making the vision loss irreversible. If inflammation involves the choroid and retina, a rather large pathologic zone appears, resulting in chorioretinal atrophy, vitreous destruction and fibrosis, imminent retinal detachment and phthisis bulbi [2, 10]. Treatment of non-infectious uveitis The goals of the therapy for uveitis are to arrest ocular inflammation and save vision in the patient. According to SUN WG guidelines, the goals of chronic uveitis treatment are to reduce the inflammatory activity and to stabilize visual function, whereas the goal of acute and recurrent uveitis treatment is to restore visual function [13]. Involvement of both local and systemic therapy into any therapeutic approach to uveitis is a must. Because autoreactive T cells, B cells, macrophages and a complex of cytokines and immunoreactive are essential for the pathogenesis of uveal inflammation, pathogenetic treatment involves glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), cytotatics and immunobiologic agents. In 1950, Gordon was the first to implement and describe the use of glucocorticoids (GCs) for treating ocular inflammation. Since that time, they have been widely used for local therapy of patients with uveitis where they can be administered via eye drops, by periocular and intravitreal injections, and as implants. In patients with inflammation refractory to local therapy, and in those with recurrent and chronic inflammation, GCs are administered systemically. They suppress proliferation of T and B cells, thus further inhibiting antibody production and interleukine secretion. Clinically, it is presented as reduced inflammatory infiltration and interstitial edema in ocular tissues. The dosage and duration of systemic therapy depends on the type and resistance of inflammation. One should however bear in mind that in complicated and refractory uveitis cases, the most effective approach involves using high-dose steroid therapy (60-80 mg/kg/day) within a month, with the following decrease by 10 mg/day every 1–2 days for acute uveitis, or by 10 mg/day every 1–2 weeks for chronic inflammation [4, 40, 42, 52]. For topical uveitis therapy, instillations of 0.1% dexamethasone phosphate, 1% prednisolone acetate, and 0.1% fluorometholone can be used. Instillations of GC are most effective in anterior uveitis patients, and have excellent penetration even in aphakic and artiphakic eyes. Although the frequency of instillation may vary from every hour to every 24 hours, we must remember that potential complications of GC instillation therapy, increased intraocular pressure (IOP) and cataractogenesis, are associated with the duration of GC treatment and total daily doses [41, 44]. The periocular route is an effective route of administration for GCs. Steroid (40 mg triamcinolone acetonide, 40-80 mg methylprednisolone acetonide, and 5 mg betamethasone dipropionate) injections into the orbital fat are performed through the upper or lower access, and are most effective in intermediate uveitis, posterior uveitis, and retinal vasculitis [40, 41, 44]. Aside from anti-inflammatory effect in anterior uveal tract, GCs cause improvement in CME and reduced production of vasculotropic agents in retinal fibroblasts and gliocytes [16, 44]. If the treatment of uveitis with instillations and periocular injections of GCs proves to be ineffective, it is reasonable administer systemic oral therapy with prednisolone or methylprednisolone, with the latter having less systemic GC side effects due to the presence of methyl group in the molecule. The initial daily prednisolone dose is 1.0–2.0 mg/kg, with the subsequent step-by-step dose reduction (one step a fortnight). Patients with acute posterior uveitis should initially receive intravenous (IV) pulse methylprednisolone therapy at a dose of 1 g/day. Later they should be switched to peroral methylprednisolone at a dose of 1.0–1.5 mg/kg/day and subsequent tapering, depending on the clinical response [3, 41, 42]. GCs can produce systemic side effects manifesting as arterial hypertension, hyperglycemia, peptic ulcer disease and gastroesophageal reflux. Topical cycloplegics are mandatory for successful treatment of anterior uveitis. They are effective not only in dilating the pupil and preventing posterior synechiae formation, but also in treating ciliary muscle spasm which presents as photophobia and ciliary pain. In the management of uveitis, the choice of cycloplegic agent (either a short-acting, 1% solution of cyclopentolate HCl, or a long-acting, 1% solution of atropine sulphate) depends on the type and duration of inflammation, amount of fibrin deposit, and iris adhesion to the anterior lens capsule [3, 15]. When topical uveitis therapy alone proves to be ineffective, different authors advocate supplementing topical uveitis therapy with systemic GCs, or cytostatics, or NSAIDs [2, 40, 41]. Thus, Foster finds an oral NSAID to be effective as an adjunct to topical therapy in uveitis, since this approach has resulted in the arrest of uveal tract inflammation in 7-10 days in 70% of such patients [15]. If the inflammation fails to resolve or become chronic, one more adjunct, a cytostatic, can be used with topical instillation of GCs and an oral NSAID [15]. Lee [41] believes that, it is a GC that should be used as the first systemic therapy for acute uveitis. If the inflammation does not resolve does not resolve within a proper period, intravitreal steroids should be administered as adjuncts [41]. The multi-center comparative study for clinical efficacy of combined methotrexate and prednisolone, the Systemic Immunosupressive Therapy for Eye Diseases Cohort (2008), found that only 36.1% of acute uveitis patients achieved long-term remission with a maintenance dose of prednisolone (10 mg/day), whereas the rest had to increase the dose or be switched to other therapies for that purpose. 76% of acute uveitis patients achieved a remission of at least a year with initial combined methotrexate and prednisolone treatment [45]. The era of cytostatic treatment for recurrent and chronic uveitis began in the mid 1980s with the treatment of JRA-associated uveitis [46]. Antimetabolites (methotrexate and azathioprine) and alkylating agents (cyclophosphamide or chlorambucil) were the first cytostatics to be produced in bulk; however, they had numerous systemic side effects associated with their non-selective influence on human cells. T and B cell (tacrolimus, mycophenolate mofetil) and Janus kinase (tofacitinib) inhibitors represent the next generation of cytostatics. Several multi-center comparison studies have investigated the treatment of uveitis with different groups of cytostatic agents, sometimes injected even subconjunctivally and intravitreally [17, 47, 48]. One must, however, remember that cytostatics should be prescribed by an immunologist, rheumatologist or general physician, and patients’ blood biochemistry should be measured trimonthly to monitor for hepatotoxicity, nephrotoxicity, hematopoietic and immune depression. NSAIDs are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) inhibitors, and can be used for uveitis locally or systemically. Indometacin and diclofenac sodium can be applied as eye drops, and selective COX-2 inhibitors are a reasonable choice for systemic treatment, since only this proinflammatory enzyme is secreted at inflammatory sites. Such COX-2 selective inhibitors as meloxicam, rofecoxib, celecoxib valdecoxib have minimal side effects and are involved in most treatment regimens used for a systemic disorder [1, 41, 49]. Combined parenteral administration of NSAIDs and antioxidants has been advocated for the management of uveitis in an attempt to potentiate the effects of each of these agents and to improve clinical efficiency [41, 49]. If no improvement is achieved after a month of treatment for uveitis, intravitreal triamcinolone acetonide (0.1 mL – 4 mg) or betamethasone dipropionate (0.1 mL – 0.5 mg) may be a reasonable step. One-time injection of these agents is usually enough to suppress inflammation for a period of 3 to 6 months. Additionally, cases of associated macular edema rapidly resolve after triamcinolone acetonide, which is supported by the increase in visual acuity [15, 40]. However, side effects of intraocular GCs (such as an increase in IOP or/and in lens opacity) have been also observed: 25% of patients required administration of hypotensive eye drops, and, in 1-2% of patients, a reduction in IOP could be achieved only with filtration surgery [3]. In 2005, a fluocinolone intravitreal sustained drug-delivery implant became the first GC implant approved for the treatment of uveitis. Yet, with application of fluocinolone implants, 75% of cases have developed ocular hypertension, and 37% of cases required filtration surgery. A new biodegradable implant which contains 0.7 mg dexamethasone has been used more recently, resulting in significant improvement in CMA and affecting IOP only in 17% of cases [50]. Immunobiologic therapy is a separate branch of medicine used in uveitis treatment and is related to monoclonal antibodies specifically binding to specific receptors and cytokines both at the surface of immunocompetent cells and in serum. Since such antibody preparations are highly specific, they have no side effects related to corticosteroids and cytostatics, and their therapeutic effect is predictable both in type and intensity [38, 51, 52]. Etanercept, involving monoclonal antibodies against TNF-?, has become the first immunobiologic agent developed for the treatment of systemic disease associated uveitis. Infliximab, the next agent of the same group, differs from etanercept in that it neutralizes the biological activity of both serum soluble and transmembane TNF-?, and has higher clinical efficiency and no side effects such as recurrence of uveitis [53]. The following generation of immunobiologic agents is chimeric (human and animal) immunoglobulins capable of inhibiting not only TNF but also IL-2, antigen CD20 and IL-1 receptors on immunocompetent cells [2, 54, 55]. Immunobiologic therapy, although comparable to pulse therapy with GKs in terms of rapidity and markedness of therapeutic effect, is associated with reduced immunity and the potential for opportunistic infections. Additionally, the scarcity of clinical trials of biological response modifiers for the treatment of uveitis and the very heterogeneity of uveitis have not yet made it possible to develop the clinical guidelines and standards of treatment. Finally, the anticytokine preparation dose providing the best balance between therapeutic effect and side effects, and optimal duration of immunobiologic therapy for uveitis are yet to be determined. Conclusion Notwithstanding a sufficient amount of data on different presentations of inflammatory uveal disease, both as a standalone inflammation and rheumatic-associated pathological condition, the general picture of the uveitides appears fragmented and poorly systematized. Furthermore, there is no uniform algorithmic approach to examination and treatment of rheumatic disease-associated uveitis. The analysis of correlations between ocular inflammation score and joint inflammation score, and between immune system status and the course of uveitis, and the course of spondyloartritis, will allow the development of the algorithmic approach to the treatment involving GKs, immune correctors and immunobiologic agents. References 1. Senchenko NL, Schuko AG, Malyshev VV. [Uveitides]. GEOTAR-Media, Moscow; 2010. 156 p. Russian. 2. Maya JR, Hanout M, May P et al. Treatment of Noninfectious Uveitis: Current Options and Agents in Development. Retinal Physician. 2013;(10):20-23. 3. Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 3rd ed. St.Louis: Mosby; 2010. 480 p. 4. Drozdova EA. [Rheumatic disease-associated uveitis: clinical features, diagnostics, immunopathogenesis and treatment] [Dr. Sc. thesis]. Cheliabinsk (Russia): Urals State Medical Academy; 2006. 319 p. Russian. 5. Ali A, Samson CM. Seronegative spondyloarthropathies and the eye. Curr Opin Ophthalmol. 2007;18(6):476–80. 6. Lee RW, Dick AD. Current concepts and future directions in the pathogenesis and treatment of non-infectious intraocular inflammation. Eye (Lond). 2012 Jan;26(1):17-28. 7. Zbitneva SV. [Incidence of ocular and ocular adnexal disorders in Ukraine]. Ukr Soc Hyg & Pub Health Org Report. 2010;(3):14-8. Ukrainian. 8. Zhaboiedov GD, Ivanova NV, Kopaienko. [Endogenous anterior uveitis and HLA-B27 antigen]. Oftalmol Zh. 2010;(3):61-6. Russian. 9. Nussenblatt RB, Gery I, Weiner HL. et al. Treatment of uveitis by oral administration of retinal antigens: results of a phase I/II randomized masked trial. Am J Ophthalmol. 1997 May;123(5):583-92. 10. Rosenberg K, Feuer W, Davis J. Ocular complication of uveitis. Ophthalmology. 2004 Dec;111(12):2299-306. 11. Ke Y, Jiang G, Sun D. et al. Ocular regulatory T cells distinguish monophasic from recurrent autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3999-4007. 12. Maca SM, Sobala A, Kahraman G et al. HLA-B27 antigen-associated acute anterior uveitis, coping mechanisms and the subjective impression of stress as a trigger: new insights. In: Program and abstracts of the Association for Research in Vision and Ophthalmology (ARVO) 2008 Annual Meeting; 2008 April 27 - May 1, 2008; Fort Lauderdale, Florida. Abstract 795. 13. Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. Am J Ophthalmol. 2005 Sep;140(3):509-16. 14. Deschenes J, Murray PI, Rao NA et al. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008 Jan-Feb; 16(1):1-2. 15. Foster CS, Vitale AT, editors. Diagnosis and treatment of uveitis. 2nd ed. New Dehli: Jaypee-Highlights; 2013. p101–295. 16. Uy HS, Christen WG, Foster CS. HLA-B27-associated uveitis and cystoid macular edema. Ocul Immunol Inflamm. 2001 Sep;9(3):177-83. 17. Huhtinen M. Acute anterior uveitis and HLA-B27: infectious background, systemic inflammation and prognosis of the patients [dissertation]. [Helsinki]: University of Helsinki; 2002. 96 p. 18. Schewitz-Bowers LР, Lee RW J, Dick DA. Immune mechanisms of intraocular inflammation. Expert Rev Ophthalmol. 2010;5(1):43–58. 19. Streilein J.W. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye (Lond). 1995;9 ( Pt 2):236-40. 20. Edelsten C, Reddy MA, Stamford MR, et al. Visual loss associated with uveitis in English primary and referral centers. Am J Ophthalmol. 2003;5:676-80. 21. Aarvak Т, Natvig JB. Cell-cell interactions in synovitis: Antigen presenting cells and T cell interaction in rheumatoid arthritis. Arthritis Res. 2001;3(1):13–7. 22. Wood B. An Overview of Uveitis and Its Management. US Pharmacist. April 1, 2011. Available from www.uspharmacist.com. [Accessed 14 April 2013]. 23. Chang JH, McCluskey PJ, Grigg JR. Recurrent hypopyon in chronic anterior uveitis of pauciarticular juvenile idiopathic arthritis. Br J Ophthalmol. 2006; 90(10):1327–8. 24. Godzenko AA. [Perspectives for treatment of rheumatic disease-associated uveitis]. Modern Rheumatol. 2011;(2):37-42. Russian. 25. Cervantes-Castaneda RA, Bhat PV, Huynh N et al. The role of azathioprine in the treatment of ocular inflammatory disease: a six month follow-up analysis. In: Program and abstracts of the Association for Research in Vision and Ophthalmology (ARVO) 2008 Annual Meeting; 2008 April 27 - May 1, 2008; Fort Lauderdale, Florida. Abstract 5822. 26. Forrester JV, Xu H, Kuffova L, Dick AD et al. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234(1):282–304. 27. Grewal I.S., Flavell R.A. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996 Oct;153:85-106. 28. Ruggieri S, Frassanito MA, Dammacco R et al. Treg lymphocytes in autoimmune uveitis. Ocul Immunol Inflamm. 2012 Aug;20(4):255-61. 29. Atan D, Fraser-Bell S, Plskova J et al. Cytokine Polymorphism in Noninfectious Uveitis. Invest Ophthalmol Vis Sci. 2010 Aug;51(8):4133-42. 30. Hecker KH, Niizeki H, Streilein JW. Distinct roles for transforming growth factor-?2 and tumour necrosis factor-? in immune deviation elicited by hapten-derivatized antigen-presenting cells. Immunology. 1999;96(3):372–80. 31. Chen L, Yang P, Zhou H. et al. Diminished frequency and function of CD4+CD25 high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3475-82. 32. Matta B, Jha P, Bora PS et al. Tolerance to Melanin-Associated Antigen in Autoimmune Uveitis Is Mediated by CD4+CD25+ T-Regulatory Cells. Am J Pathol. 2008 Nov; 173(5): 1440–1454. 33. Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981 May 1; 153(5): 1058–67. 34. Elschnig A. Studien sur sympathischen Ophthalmie: Die Antigene Wirkung der Augenpigmente. Graefes Arch Ophthalmol. 1910;67:509-46. 35. Wacker WB, Barbee JY, Macdonald R. Experimental allergic uveitis III. Manifestations produced in the guinea pig by immunization with homologous retina. Invest Ophthalmol Vis Sci. 1969;8(4):381-91. 36. Rai G, Saxena S, Kumar H et al. Human retinal S-antigen: T cell epitope mapping in posterior uveitis patients. Exp Mol Pathol. 2001 Apr;70(2):140-5. 37. Hankey DJ, Lightman SL, Baker D et al. Interphotoreceptor retinoid binding protein peptide-induced uveitis in B10.RIII mice: characterization of disease parameters and immunomodulation. Exp Eye Res. 2001; 72(3):341-50. 38. Shao H, Lei S, Sun SL et al. Conversion of Monophasic to Recurrent Autoimmune Disease by Autoreactive T Cell Subsets. J Immunol. 2003 Nov 15;171(10):5624-30. 39. Lee RW, Schewitz LP, Nicholson LB et al. Steroid refractory CD4+ T cells in patients with sight-threatening uveitis. Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4273-8. 40. Couch SM, Bakri SJ. Intravitreal triamcinolone for intraocular inflammation and associated macular edema. Clin Ophthalmol. 2009;3:41-7. 41. Lee FF, Foster CS. Pharmacotherapy of uveitis. Expert Opin Pharmacother. 2010 May;11(7):1135-46. 42. Nguyen QD, Hatef E, Kayen B et al. A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology. 2011 Jan;118(1):184-90. 43. Savko VV, Konovalova NV, Naritcyna NI. [Features of treatment of chronic uveitis]. Oftalmol Zh. 2009;(3):83-6. Russian. 44. Bae J.H., Lee C.S., Lee S.C. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina. 2011;31(1):111-8. 45. Kempen JH, Daniel E, Gangaputra S et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008 Jan-Feb;15(1):47-55. 46. Galor A, Jabs DA, Leder HA. Comparison of Antimetabolite Drugs as Corticosteroid-Sparing Therapy for Noninfectious Ocular Inflammation. Ophthalmology. 2008 Oct;115(10):1826-32. 47. Nguyen QD, Ibrahim MA, Watters A. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013 Feb 11;3(1):32. 48. Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure of intolerance in the treatment of scleritis or uveitis. Ophthalmology. 2008 Aug;115(8):1416-21. 49. Absalikova DK, Mal’khanov VB. [Clinical and laboratory validation of concomitant NSAIDs and antioxidant therapy for endogenous uveitis]. ONU Report. 2011;133(14):12-5. Russian. 50. Lowder C, Belfort R Jr, Lightman S et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011 May;129(5):545-53. 51. Taylor SR, Banker A, Schlaen A et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013 Nov-Dec;33(10):2149-54. 52. Okada AA. The dream of biologics in uveitis. Arch Ophthalmol. 2010 May;128(5):632-5. 53. Smith JR, Levinson RD, Holland GN et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001 Jun;45(3):252-7. 54. Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007 Nov;18(6):481-6. 55. Tomkins-Netzer O, Taylor SR, Lightman S. Can Rituximab Induce Long-Term Disease Remission in Patients with Intra-Ocular Non-Infectious Inflammation? Ophthalmologica. 2013;230(3):109-15.
|