J.ophthalmol.(Ukraine).2015;5:42-45.
|
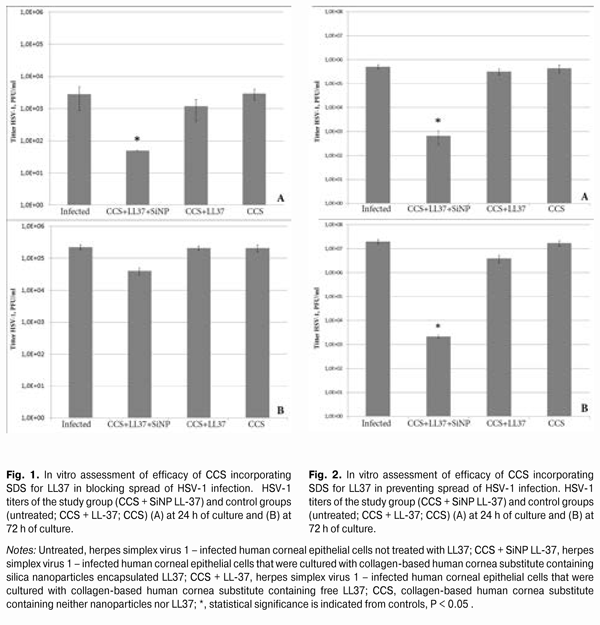
https://doi.org/10.31288/oftalmolzh201554245 Antiviral properties of collagen-based corneal substitute incorporating sustained delivery system for anti-infective peptide LL37 O.I. Buznyk,1 MD, Cand. Sc. (Med), C.-J. Lee,2 PhD, M.M. Islam,3 MSc, N.V. Pasyechnikova,1 MD, Dr. Sc. (Med), M. Griffith, 2 PhD 1 Filatov Institute of Eye Diseases & Tissue Therapy, Ukraine (Odessa), 2 Linkoping University (Linkoping, Sweden), 3 Karolinska Institute (Stockholm, Sweden) E-mail: a_buznik@bk.ru Financial disclosure: This research has been partially supported by a grant from the Swedish Institute in Stockholm. Purpose: To investigate in vitro the anti–Herpes Simplex Virus (HSV)-1 properties of the collagen-based corneal substitute (CCS) incorporating the sustained delivery system for anti-infective peptide (AIP) LL37. Methods: AIP LL37 was encapsulated into silica nanoparticles (SiNPs) under magnetic stirring. SiNPs with LL37 were then introduced into the CCS at the time of its fabrication by creating interpenetrating networks of type I collagen and phosphorylcholine. The anti-HSV-1 properties of the composite CCS were assessed by counting plaque forming units (PFU). Results: When the CCS incorporating the SDS for LL37 was added to the culture of human corneal epithelial cells (HCECs), virus concentration in the 24-h and 72-h culture media was less than 50 PFU/mL and 39333.3±9291.6 PFU/mL, respectively. Concentration of HVS-1 in the 24-h and 72-h culture media for HCECs without CCS-based treatment was 2800±1928.7 PFU/mL and 221666.7±36855.6 PFU/mL, respectively (P24h = 0.039, Р72h = 0.063). Conclusion: The CCS incorporating the SDS for LL37 was efficacious within 24 h of culture, when used to block the spread of HSV-1 infection in vitro in HCECs. Additionally, the CCS remained efficacious at 24 h and 72 h of culture, when used for the prophilaxis of HSV-1 infection. Keywords: artificial cornea, sustained delivery system, LL37, herpes simplex virus Introduction The limited efficacy of existing anti-infective agents for treatment of ocular infections [1] coupled with a low efficacy of corneal grafting in infectious keratitis and its sequelae [2, 3] highlights the urgent need for alternative methods of treating infectious corneal diseases. One of the alternatives involves using peptide LL37, which has been well investigated, with its potent antibacterial and antiviral activity found to be preserved following the introduction of the peptide into various sustained drug delivery systems [4-7]. Recent tissue engineering advances made it possible to synthesize collagen-based human cornea substitutes having optical, chemical and mechanical properties close to those of human corneas [8]. Experimental and clinical studies demonstrated a high level of biocompatibility of collagen-based implants, requiring no immune suppression [9-11]. Previously, we have synthesized a human collagen corneal substitute (CCS) incorporating the sustained delivery system (SDS) for Anti-infective Peptide (AIP) LL37 based on SiO2 nanoparticles (SiNPs) [12]. This composite implant has been shown to have satisfactory levels of mechanical and optic characteristics, with gradual release of LL37 from SiNPs during three weeks. The purpose of the study was to investigate in vitro the anti-Herpes Simplex Virus (HSV)-1 properties of the CCS incorporating the SDS for AIP LL37. Materials and Methods The study was performed at the Division of Cell Biology, Department of Clinical and Experimental Medicine, Linkoping University (Sweden). Fabrication of sustained delivery system for anti-infective peptide LL37 The process of fabrication of a SDS for AIP LL37 involved encapsulation of LL37 into SiNPs under magnetic stirring. A detailed protocol for the fabrication of this SDS has been described previously [13]. Fabrications of collagen-based corneal substitutes with or without sustained delivery system for LL37/ free LL37 The CCS was fabricated by creating interpenetrating networks of porcine type I acidic atelocollagen solution (Nippon Meat Packers, Inc., Tokyo, Japan) and 2-methacryloyloxyethyl phosphorylcholine (MPC) (Paramount Fine Chemicals Co. Ltd, Dalian, China) [10]. Briefly, 500 mg of 14% (wt/wt) porcine type I acidic atelocollagen solution was buffered with 150 ?L of 0.625 M morpholinoethanesulphonic acid (MES, EMD Chemicals, Canada) buffer in a two-syringe mixing system. Two hundred microliters of MPC solution with 0.625 M MES buffer were then added. The MPC:collagen ratio (wt/wt) was 1:2. Thereafter, poly(ethylene glycol) diacrylate PEGDA (Sigma Aldrich Sweden AB, Stockholm, Sweden) was added into the mixing system. The PEGDA:MPC ratio (wt/wt) equaled 1:3. When a composite implant was to be synthesized, LL-37 (free or SiNP encapsulated) was added into solution at this stage, with 300 ?L of free LL-37 solution (500 ?g/mL) or 300 ?L of LL-37 SiNPs solution (7 mg/mL) introduced into the mixing system. Then, calculated amounts of 4% (wt/vol) ammonium persulphate (APS; Sigma-Aldrich) buffered with MES and 2% (wt/vol) N,N,N ,N -tetramethyl ethylene diamine (TEMED; Sigma-Aldrich) buffered with MES were added into the mixing system. The APS:MPC ratio (wt/wt) was 0.03:1, the APS:TEMED equaled 1:0.77. After thorough mixing, calculated amounts of N-hydroxyl-succinimide (NHS, Sigma Aldrich) (10% (w/vol) in MES) and N-(3-Dimethylaminopropyl)-N?-ethylcarbodiimide hydrochloride (EDC; Sigma-Aldrich) (5% (w/vol) in MES) were added and the reactants were thoroughly mixed at 0°С. The EDC:collagen-NH2 ratio was 0.7:1 (mol/mol), while the EDC:NHS ratio was 2:1 (mol/mol). The final mixed solution was immediately cast between two glass plates with 500 ?m spacers. The hydrogels were cured at 100% humidity under nitrogen at room temperature for 16 h. After demoulding, they were washed thoroughly with 10 mM phosphate-buffered saline (PBS) and then stored in PBS at 4°С. Assessment of antiviral properties of collagen-based corneal substitute incorporating sustained delivery system for peptide LL37 Assessment of efficacy of CCS incorporating SDS for LL37 in blocking spread of HSV-1 infection In the first part of the experiment, immortalized human corneal epithelial cells (HCECs) [14] were cultured until 90% confluence in 96-well tissue culture plates supplemented with Keratinocyte Serum Free Medium (KSFM) containing L-glutamine, human epidermal growth factor (EGF) and bovine pituitary extract (BPE; Life Technologies Europe BV). Then the HCECs were exposed to HSV-1 strain F at a multiplicity of infection (MOI) of 0.05 for 1 hour at 37°С. After being washed thoroughly with KSFM, the cells were supplemented with fresh KSFM and composite implants incorporating LL-37 SiNPs, and cultured for 72 hours. Other HCECs ((1) those non-treated; (2) those to which CCS without LL37 was added; and (3) those to which CCS with free LL37 was added) were also cultured for 72 hours; they were used as controls. Samples of culture media were taken at 24 h and 72 h to assess the concentration of HSV-1 by counting PFUs. Assessment of efficacy of CCS incorporating SDS for LL37 in preventing spread of HSV-1 infection In the second part of the experiment, HCECs were exposed to HSV-1 (MOI = 0.1) at 24 h after their co-culture either with the CCS incorporating the SDS for LL37, or with the CCS without LL37, or with the CCS with free LL37. After being incubated with HSV-1 at 37°C for 1 hour, the cells were washed thoroughly, overlaid with KSFM and the CCS with SDS for LL37, and cultured for another 48 h. Samples of culture media were taken to assess the concentration of HSV-1 by counting PFUs. PFU counting method Various serial dilutions (10-1 – 10-6) of the culture media obtained were added onto 80% confluent African green monkey kidney epithelial Vero cells (American Type Culture Collection, USA) and incubated at 37°C for 1 hour. After removal of the culture media, the cells were overlaid with Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Europe BV, Sweden) containing 5% FBS and 1% agarose and cultured for 72 hours. Thereafter, they were fixed with 10% formaldehyde and stained with 0.5% crystal violet. The number of plaques formed was counted. Statistics The nonparametric Kruskal-Wallis test was used to assess differences between the study group (CCS with SDS for LL37) and control groups ((1) CCS without LL37, (2) CCS with free LL37, and (3) infected untreated HCECs). Each group involved 4 samples. Statistical significance was set at P less than 0.05. Results Assessment of efficacy of CCS incorporating SDS for LL37 in blocking spread of HSV-1 infection At 24 hours postinoculation, the CCS incorporating the SDS for LL37 demonstrated better anti-HSV1 activity than controls (infected untreated cells and the cells that had been cultured with the CCS containing free LL37). HSV-1 titers were 2800±1928.7 PFU/mL, decreased to 1167.7±763.7 PFU/mL, and were less than 50 PFU/mL (P = 0.039), respectively, in infected untreated HCECs, and in the presence of the CCS that contained free LL-37, and in the presence of the CCS that incorporated LL-37 SiNPs (Fig. 1A).
At 72 hours postinoculation, the anti-HSV1 activity of composite implants was modest, with a still substantial, but not statistically significant difference in antiviral activity between them and controls (P = 0.063). HSV-1 titers were 221666.7±36855.6 PFU/mL, almost did not change (206666.7±30550.5 PFU/mL), and were 39333.3±9291.6 PFU/mL (P = 0.039), respectively, in infected untreated HCECs, and in the presence of the CCS that contained free LL-37, and in the presence of the CCS that incorporated LL-37 SiNPs. CCS alone showed no intrinsic antiviral activity (Fig. 1B). Assessment of efficacy of CCS incorporating SDS for LL37 in preventing spread of HSV-1 infection Adding the CCS incorporating LL-37 SiNPs to HCECs 24 h before inoculating these HCECs with HSV-1 resulted in a more pronounced protective effect of LL-37 24 h and 72 h post-inoculation compared with the CCS that contained free LL-37 and with infected untreated HCECs. Twenty-four hours and 72 h post-inoculation, virus titers in infected untreated HCECs were 5х105±1х105 PFU/m and 19.3х106±4.3х106 PFU/m, respectively, versus 3.1х105±0.8х105 PFU/m and 3.8х106±1.3х106 PFU/m, respectively, in the presence of the CCS that contained free LL-37. At the same time points, virus titers in the study group were 666.7±381.9 PFU/m and 2133.3±321.4 PFU/m (P24h = 0.032, P72h = 0.027, Fig. 2), respectively, whereas CCS alone showed no intrinsic antiviral activity. Discussion In this paper, for the first time, to the best of our knowledge, we have proved the anti-HSV-1 activity of the composite graft which can be used in keratoplasty for corneal infectious diseases simultaneously as a corneal substitute and as a carrier of the anti-infectious agent. We have shown that, when added to collagen solution at the time of CCS synthesis, the SiNp-based sustained system developed to deliver anti-infective peptide LL37 does not lose its beneficial features, and the peptide keeps exerting the effect on the virus. The greatest LL37effect was observed when the peptide was used as the infection prophylaxis agent. When the implants incorporating the system for LL37 delivery were used to block viral spreading in cells, the antiviral effect was significant compared to the controls, but somewhat reduced at 72 h postinoculation. Taking into account that conventional keratoplasty has shown a low efficacy in the treatment of HSK [3], such composite implants are potential alternatives to donor corneas for restoring vision to herpes simplex keratitis (HSK) patients. Additionally, of interest is the use of the CCS with SDS for LL37 as a re-infection prophylaxis agent in keratoplasty for corneal opacification following herpetic keratitis. Such implants can be enhanced through improving their mechanical characteristics and transparency, and by increasing their medication content. Taking into account that, when introduced into the CCS at the time of CCS synthesis, the system developed for sustained delivery of LL37 did not lose its antiviral features, one may suppose that the CCS incorporating the SDS for the anti-infectious peptide will maintain also its antibacterial characteristics which have been tested previously [5]. The findings obtained provide promise for further investigation of corneal substitutes incorporating a sustained release medication delivery system as a means of treating ocular viral and bacterial infections. References 1. Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J Antibiot (Tokyo). 2010; 63(8):423–430. 2. Lyall DA, Tarafdar S, Gilhooly MJ, Roberts F, Ramaesh K. Long term visual outcomes, graft survival and complications of deep anterior lamellar keratoplasty in patients with herpes simplex related corneal scarring. Br J Ophthalmol. 2012;96:1200-1203. 3. Sharma N, Sachdev R, Jhanji V, et al. Therapeutic keratoplasty for microbial keratitis. Curr Opinion Ophthalmol. 2010;21:293-300. 4. Islam MM, Mondal D, Griffith M, Buznyk OI. Anti-infective peptide LL37 sustained delivery system – a potential novel treatment method of ocular infections. Report 2. Anti-viral properties of silica nanoparticle encapsulated LL37. Oftalmol Zh. 2014;3:53-7. Ukrainian. 5. Buznyk OI, Pasyechnikova NV, Iakymenko SA, Moloda AL, Islam MM, Griffith M. Anti-infective peptide LL37 sustained delivery system – a potential novel treatment method of ocular infections. Report 3. Anti-microbial properties of silica nanoparticle encapsulated LL37. Oftalmol Zh. 2014;5:4-8. In Ukrainian. 6. Gordon YJ, Huang LC, Romanowski EG, et al. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30(5):385–394. 7. Izquierdo-Barba I, Vallet-Reg? M, Kupferschmidt N, et al. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials. 2009;30(29): 5729-5736. 8. Griffith M, Polisetti N, Kuffova L, et al. Regenerative approaches as alternatives to donor allografting for restoration of corneal function. Ocul Surf. 2012;10(3):170-183. 9. Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. 10. Liu W., Deng C, McLaughlin CR, et al. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551-1559. 11. Pasyechnikova N, Vit V, Leus M, et al. Collagen-based bioengineered substitutes of donor corneal allograft implantation: assessment and hypotheses. Med Hypothesis Discov Innov Ophthalmol. 2012;1(1):10-13. 12. Buznyk OI, Pasyechnikova NV, Iakymenko SA, Islam MM, Griffith M. Collagen-Based Corneal Substitutes with Incorporated Anti-infective Peptide LL37 Sustalined Deivery System. Oftalmol Zh. 2015;1:110-114. 13. Buznyk OI. Anti-infective peptide LL37 sustained delivery system – a potential novel treatment method of ocular infections. Report 1. Testing of different carriers of LL37. Oftalmol Zh. 2014;2:17-21. Ukrainian. 14. Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614-621. 15. Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357-364.
|