J.ophthalmol.(Ukraine).2016;2:23-27.
|
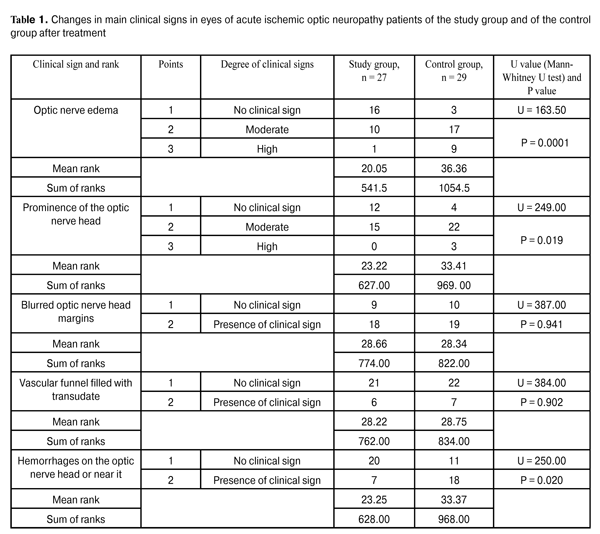
https://doi.org/10.31288/oftalmolzh201622327 On the correction of altered ocular hemodynamics in acute ischemic optic neuropathy V.V. Savko Snr, Dr Sc (Med) V.V. Savko Jnr, Cand Sc (Med) Filatov Institute of Eye Disease and Tissue Therapy Odessa (Ukraine) E-mail: valchuk2001@ukr.net Background: Vascular optic nerve disorders are the second most common incapacitating ocular condition (19%) in Ukraine. Inadequate efficacy of available therapies calls for the search of medications that would improve ocular hemodynamics in this disease. Purpose: To investigate the influence of sulodexide on ocular hemodynamics in acute ischemic optic neuropathy. Materials and Methods: Twenty-seven acute ischemic optic neuropathy patients (27 eyes) underwent clinical studies at baseline and after 15-day adjuvant treatment with Vessel due F (2.0 mL intramuscular injection daily). The control group included 29 acute ischemic optic neuropathy patients (29 eyes) treated by therapy without adjuvant Vessel due F. All patients underwent Doppler ultrasonography of the ophthalmic artery with LOGIQ 3 Expert (GE, USA) apparatus. Results: After treatment, Doppler ultrasonography indices improved only in patients of the study group, with 28% increased mean peak blood flow velocity and 31% increased mean minimum blood flow velocity in the ophthalmic artery, and 15% decreased vascular resistance index. No significant changes in these parameters were observed in the controls. Conclusion: The use of adjuvant Vessel due F corrected the altered regional ocular hemodynamics and resulted in improved treatment outcomes in acute ischemic optic neuropathy patients. Key words: acute ischemic optic neuropathy, Doppler ultrasonography, ophthalmic artery, Vessel due F Introduction Ischemic optic neuropathy is one of the most severe ocular disorders. The disease is associated with abrupt and progressive sight-threatening deterioration in visual function, and, if untreated, can lead to the development of either partial or total optic nerve atrophy [1-4]. In recent years in Ukraine, it has gone from the fifth to the second most common cause (19%) of ocular incapacitation [5]. This optic nerve disorder is mostly secondary to atherosclerosis, hypertensive disease, diabetes mellitus and rheumatism [1, 6-8]. The development of ischemic optic neuropathy is underpinned by alteration in ocular hemodynamics secondary to reduced or interrupted arterial blood supply of the eye. Stenosis or occlusion of the arterial vessels supplying the optic nerve, and the resulting imbalance between perfusion pressure in these vessels and intraocular pressure contribute to the development of ischemic changes in optic nerve tissues [2, 4, 6, 9]. Another important component of the pathogenesis is deterioration of rheological blood properties, functional state of the erythrocyte membrane, blood plasma composition, deformation and aggregation properties of erythrocytes, which has a high potential for thrombosis. Thrombosis, in its turn, leads to vessel occlusion and deteriorated blood supply of the optic nerve, which is followed by nerve fiber death due to the appearance of foci of softening and circumscribed gliosis in these fibers and growth of the connective tissue [1, 4, 10, 11]. Based on the course of the disease, ischemic optic neuropathy (ION) is grouped into chronic and acute ION. In our point of view, the ION classification developed by Eremenko [9] is the most rational one from the practitioner’s viewpoint. It distinguishes vascular sector papillitis, vascular papillitis, ischemic edema of the optic nerve head (ONH) and vascular retrobulbar neuritis as four clinical variants of the course of the ION. In vascular sector papillitis, a cyanotic-pinkish or waxy discoloration of one edematous ONH sector with blurred ONH margins is observed. In vascular papillitis, a bluish or waxy discoloration of a prominent ONH with blurred margins is observed, and the vascular funnel is noted to be filled with a clear transudate. Additionally, point- or stripe-shaped hemorrhages may be revealed on the ONH or near it, in the edematous retina. In ischemic optic nerve head edema, ophthalmoscopy reveals a prominent and edematous ONH with diffuse pallor and blurred margins. In vascular retrobulbar neuritis, an ONH is slightly edematous and hyperemic or remains unchanged [9]. According to Eremenko, initial forms of acute ION (vascular sector papillitis, vascular papillitis, and ischemic edema of the ONH) represent not nosologic forms of the disease, but sequential stages of the disease process, with the severity of ischemia varying from one form of the disease to another. Ischemic edema of the ONH is characterized by the most severe changes, and is usually associated with central retinal artery occlusion and branch retinal arteriole occlusion, which worsens the prognosis for recovery of visual function. Treatment of this disorder is based on improvement in main blood flow and in collateral blood flow (vasodilators), rheological blood properties (anticoagulants, rheology enhancers, thrombolytics) with the use of hyperosmotic agents, antioxidants, calcium channel antagonists, and angioprotectors [4, 10, 12-14]. However, despite a wide range of available medications, treatment of acute ION is still a challenge, as no reasonably efficacious treatment methodology has been introduced [13]. Inadequate efficacy of available therapies has drawn our attention to an antithrombotic agent, sulodexide (Vessel due F, Alfa Wassermann, Italy; Ukrainian Medical Register Certificate, №UA/8123/01/01). It is classified as antithrombotic, anticoagulant, and fibrinolytic. Additionally, it protects blood vessels, and has been used widely in the treatment of altered cerebral circulation, cerebrovascular disorders, occlusion of peripheral arteries, phlebopathy and deep vein thrombosis, diabetic angiopathy and antiphospholipid syndrome. Ophthalmologic literature is scarce on the use of sulodexide for diabetic retinopathy. In these reports, it has been noted that the agent can prevent formation of hard exudates and retinal hemorrhages and reduce macular edema [15-17]. Although alteration in ocular hemodynamics is a key pathogenetic factor in acute ION, the influence of sulodexide on this hemodynamics, to the best of our knowledge, has not been investigated until now. The study purpose was to investigate the influence of sulodexide on ocular hemodynamics in acute ischemic optic neuropathy. Materials and Methods Fifty six patients (56 eyes) with acute ION (age, 59 to 72 years; 35 men and 21 women) were included into the study. Thirty nine and 17 of them had general atherosclerosis and hypertensive disease, respectively. Patients were divided into the study group (27 patients; 27 eyes) and the control group (29 patients; 29 eyes). Vascular papillitis and ischemic edema of the ONH were observed in 5 patients and 22 patients, respectively, of the study group, and in 6 patients and 23 patients, respectively, of the control group. Patients of the groups were comparable in terms of the main clinical signs of the disease: the degree of optic nerve edema (р=0.628), the degree of prominence of the ONH (р=0.512), blurred optic nerve head margins (р=0.329), presence of the vascular funnel filled with transudate (р=0.386), presence of hemorrhages on the ONH or near it (р=0.753), and visual acuity (р=0.474). Main clinical signs of acute ION were scored using a special scale [1]. Patients of both groups were treated with vasodilator therapy (NO-SPA; oxybral; papaverine, 2.0 ml intramuscular; and cavinton, 5.0 ml intramuscular), anticoagulants (fraxiparine, 0.3 ml parabulbar), angioprotectors (dicynone, 0.5 ml parabulbar and 2.0 ml intramuscular; ascorbic acid, 2.0 ml intramuscular), and dehydration therapy (furosemide, by mouth or 2.0 ml intramuscular). Additionally, patients of the study group were treated with 600 lipoprotein lipase units of sulodexide (Vessel due F, 2.0 ml intramuscular) daily during 15 days. Patients tolerated this medication well, with no side effects being observed. Each patient underwent visual acuity measurement, perimetry, biomicroscopy and ophthalmoscopy. Doppler ultrasonography with LOGIQ 3 Expert (GE, USA) apparatus and 4-MHz transducer was used to examine regional ocular hemodynamics. The following blood flow parameters were measured in the ophthalmic artery: Vmax, systolic peak velocity; Vmin, end-diastolic (minimum) velocity, and RI, Resistivity Index, reflecting the state of the vascular resistance to blood flow distal to the measurement point. In Doppler ultrasonography studies, the eyes under investigation were compared with 20 fellow eyes without ocular nerve disorder. All statistical analyses were done using SPSS 11.0 (SPSS, Inc, Chicago, IL) software. Quantitative variables were compared using Student’s t test. A nonparametric method for independent samples (Mann–Whitney test) was used for intergroup comparison of the parameters characterizing individual clinical sign scores. Results and Discussion The mean visual acuity improved significantly from 0.11 ± 0.01 to 0.27 ± 0.03 (р ? 0.001) in eyes of the study group and insignificantly, from 0.09 ± 0.01 to 0.16 ± 0.04 (р ? 0.05), in eyes of the control group. Additionally, the mean total visual field (from eight meridians) improved from (428.3° ± 8.2°) to (483.7° ± 6.1°) (р?0.001) in eyes of the study group and from (434.2° ± 5.8°) to (467.6° ± 7.2°) (р?0.001) in eyes of the control group. Table 1 presents the changes in the clinical sign scores in eyes of both groups after treatment.
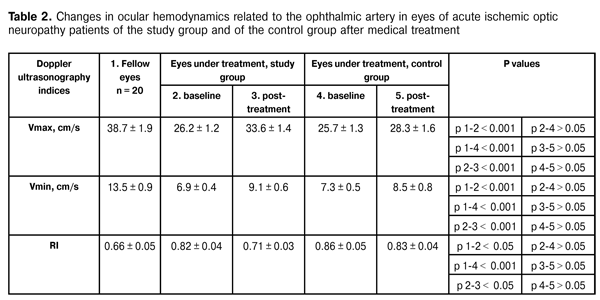
It is noteworthy that the reduction in clinical sign scores for eyes of the study group was far more significant than that for eyes of the control group, which was evidenced by lower mean ranks in the former group. Thus, the optic nerve edema score in the study group was significantly lower than that in the control group (р = 0.0001). Additionally, patients of the study group had significantly less prominence of the ONH (р = 0.019) and a more significant reduction in hemorrhages on the ONH or near it (р = 0.020) compared to these measures in the controls. Table 2 presents the results of Doppler ultrasonography studies of ocular hemodynamics related to the ophthalmic artery in eyes of both groups.
The analysis of the results shows that, mean baseline Vmax value in eyes under investigation was significantly lower than that in fellow eyes ((26.2 ± 1.2) cm/s vs (38.4 ± 1.9) cm/s in patients of the study group and (25.7 ± 1.3) cm/s vs (38.4 ± 1.9) cm/s in patients of the control group, i.e., 32% and 34% lower). Additionally, mean baseline Vmin value in eyes under investigation was also significantly lower than that in fellow eyes (6.9 ± 0.4 cm/s vs 13.5 ± 0.9 cm/s in patients of the study group and 7.3 ± 0.5 cm/s vs 13.5 ± 0.9 cm/s in patients of the control group, i.e., 49% and 46% lower). Moreover, mean baseline RI value in eyes under investigation was significantly higher than that in fellow eyes (0.82 ± 0.04 vs 0.66 ± 0.04 in patients of the study group and 0.86 ± 0.05 vs 0.66 ± 0.04 in patients of the control group, i.e., 24% and 29% lower). The intergroup difference in baseline Doppler ultrasonography indices between study group and controls was found to be insignificant. Following treatment, only intragroup differences in Doppler ultrasonography indices among patients of the study group were found to be significant: mean Vmax value and mean Vmin value increased to 33.6 ± 1.4 cm/s (28%), and 9.1 ± 0.6 cm/s (31%), respectively, whereas RI value decreased to 0.71 (15%). In patients of the control group, these parameters did not change significantly over time of treatment. Therefore, increase in systolic peak velocity and end-diastolic (minimum) velocity in the ophthalmic artery, and decrease in vascular resistance to blood flow demonstrate improvement in posterior ocular blood flow in patients with acute ischemic optic neuropathy treated with adjuvant Vessel due F. Conclusion First, inclusion of adjuvant Vessel due F therapy demonstrated a superior corrective effect on the altered regional ocular hemodynamics, with increase in systolic peak velocity and end-diastolic (minimum) velocity (28% and 31%, respectively) in the ophthalmic artery, and 15% decrease in vascular resistance to blood flow in patients with acute ischemic optic neuropathy. Second, the use of this adjuvant therapeutic agent resulted in improved treatment outcomes, with significant improvement in visual acuity, visual field performance and reduction in the main clinical signs of the disease. References
|