J.ophthalmol.(Ukraine).2016;5:22-28.
|
https://doi.org/10.31288/oftalmolzh201652228 Changes in visual acuity in patients with dry form of age-related macular degeneration after low-energy light therapy and medication A.M. Sergienko 1, Dr. Sc. (Med.), Prof. N.O. Dzyuba 2, MD 1 Professor Sergienko`s Eye Clinic, Vinnitsa, Ukraine 2 Kyiv city center of the diagnosis and treatment of vascular degenerative eye diseases, Kyiv city clinical hospital № 9, Kyiv, Ukraine
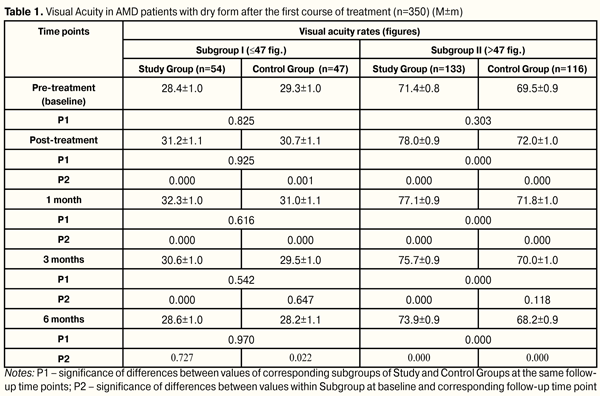
Introduction. Age-related macular degeneration (AMD) leads to partial or complete loss of central vision, causing disability of patients. The current problem explains completely the search for new methods of influence on the visual analyzer in patients with AMD. The рurpose. To study the changes in visual acuity in patients with the dry form of AMD after two courses of low-energy light therapy and medication. Material and methods. Study Group was made up of 115 patients (187 eyes), which took two courses of low energy light therapy (LELT) in combination with two courses of medication in the hospital for 10 days each. Control Group consisted of 95 patients (163 eyes), which passed only two courses of medication in the hospital for 10 days each. Time points of follow up were before treatment (baseline), after treatment, 1, 3, and 6 months after the first course of treatment. Afterwards, the second treatment course was performed with the same time points. Visual acuity testing was performed using ETDRS tables (number of characters). Depending on the visual acuity (VA), Study Group was divided as follows: Subgroup I, 54 eyes ? 47 fig.; Subgroup II, 133 eyes > 47 fig. Control Group: Subgroup I, 47 eyes ? 47 fig.; Subgroup II, 116 eyes > 47 fig. Results and their discussion. After completing two courses of treatment,VA rate improvement was by 21.6% better in Study Subgroup I patients than in Control Subgroup I ones, i.e. (22 eyes (40.7%) vs. 9 eyes (19.1%), respectively; VA rate stabilization was noted in 6 eyes (11.1% cases) of Study Subgroup I patients. Overall, treatment was more successful by 32.7% in Study Group as compared with Control Group, i.e. 28 eyes (51.8%) vs. 9 eyes (19.1%), respectively. Decrement in VA rates was less by 32.8% in Study Group than in Control Group, i.e. 26 eyes (48.1 %) vs. 38 eyes (80.9 %), respectively. After completing two courses of treatment in patients of Study and Control Subgroups II, VA improvement rates were higher by 79.8% in Study Group patients than in those of Control Group, i.e. 113 eyes (85.0%) vs. 6 eyes (5.2%), respectively; VA stabilization rates were higher by 4.6% in Control Group patients than in those of Study Group, i.e. 15 eyes (12.9%) vs. 11 eyes (8.3%), respectively. Overall, treatment success was higher by 75.2% in Study Group than in Control one, i.e. 124 eyes (93.3%) vs. 21 (18.1%), respectively. Conclusions. 1.It was found that AMD patients with low vision had stabilization of VA rates (pre-treatment (28.6±1.0) figures, post-treatment (28.7±1.1) figures, р=0.496) after two joint courses of low-energy light therapy and medication; meanwhile, patients receiving medication only therapy had significant decrement in visual acuity from (29.3±1.0) to (26.2±1.2) figures (р=0.000). 2.It was revealed that, after two joint courses of low-energy light therapy and medication, AMD patients with high VA rates had significant increase of VA rates from (71.4±0.8) figures at baseline to (76.6±0.9) figures at 12 months, (р=0.000); while VA rates decreased from (69.5±0.9) to (66.3±0.9) (р=0.000), respectively, in patients receiving medication only therapy. 3.It was noted that treatment of AMG patients both with poor and high VA rates who underwent two joint courses of low-energy light therapy and medication was more successful than in those who received two courses of medication only therapy. The difference between them was 32.7% (14.0 ? 48.15. р=0.001) for poor VA rates and 75.2% (65.4 ? 81.9, р<0.000) for high VA rates.4.Success of low-energy light therapy in combination with medication course in AMD patients depends on baseline VA rates: the higher visual acuity is, the more successful is the treatment. Key words: vision acuity, low-energy light therapy, medication, age-related macular degeneration Introduction Over the last years a significant increase in incidence of age-related macular degeneration (AMD) among working age and general Ukraine’s population has been marked [3, 6, 7, 12, 13, 15]. One of the main functions of the eye is visual acuity. When AMD develops, visual acuity decreases gradually; and developed dry and especially exudative forms of AMD can lead to partial or complete loss of central vision. This results in permanent partial disability and invalidity of the population which makes this problem of social and economic importance [4, 5, 6, 7, 10, 12, 14]. The current problem explains completely the search for new pathogenetically targeted methods of influence on visual analyzer in AMD patients with a view to preserve visual acuity as main function of the eye. One of the possible factors of influence on visual acuity in AMD patients is light. Authors have proved the positive effect of monochromic light on different ocular functions [2, 8, 9, 11, 16, 17]. Golovin-Sivtsev decimal-based table with 0.1 differences between rows is commonly used in Ukraine for testing visual acuity. However, this method is not used in international multi-centre trials since it has poor accuracy. Instead of this, a standardized visual acuity test using LogMARETDRS tables has been accepted (Early Treatment Diabetic Retinopathy Study; a test has been developed for visual acuity assessment after panretinal laser photocoagulation in diabetic retinopathy patients). This test has been designed to avoid errors in tests using Snellen chart with Sloan letters and to measure statistically significant visual acuity. LogMARETDRS charts are characterized by: 1.The same number of optotypes in each row (5 letters on each line) 2.The same logscale interval between letters 3.The same 0.1 log interval between lines 4.Certain lines are balanced on figure complexity There are three standard LogMARETDRS charts: R, 1 and 2. These charts have been designed to avoid memorizing letters. LogMARETDRS charts must have standard illumination for correct assessment. Visual acuity testing procedure using these charts is fast and clear [18, 19]. LogMARETDRS is the most accurate among visual acuity testing charts and can be used with different viewing distances. The value of each letter is 0.02 logs; so, visual acuity can be measured quite accurately even when a patient cannot see all the letters in the line [1]. The purpose of the present study was to study changes in visual acuity of patients with age-related macular degeneration after two courses of low-energy light therapy and medication. Material and Methods We followed up 210 patients (350 eyes) with dry form AMD. Inclusion criteria were the presence of a) round or oval-based focus of de- or hyperpigmentation without retinal pigment epithelium (RPE); b) soft shallow and drain drusen; c) areas of RPE de- hypo- and hyperpigmentation. Study Group (SG) comprised 115 patients (187 eyes) who underwent two courses of low-energy light therapy (LELT) in combination with two medication courses under inpatient treatment for 10 days each. Control Group (CG) consisted of 95 patients (163 eyes) undergone only two courses of inpatient medication treatment for ten days each. The interval between courses was 6 months. Time points of follow up were before treatment (baseline), after treatment, 1, 3, and 6 months after the first course of treatment. Afterwards, the second treatment course was performed with the same time points. SpektraLight unit (Version MARK III) (Canada, Vision Aid Inc., Winnipeg, MB, Canada, together with StarFishLtd., Victoria, BC) was used to perform LELT. The procedure of transpupillary exposure of the retina was carried out using monochromic impulse light of green, red, and infra-red spectra with 2х10-6 J energy, 10 mc impulse duration, 30 mc pulse repetition rate, and 8.3 mW/cm2 power density on the cornea. The treatment course included ten sessions 5 minutes each during 10 days (one session a day). The medication treatment included emoxypine, parabulbar injection in a dose of 0.5 ml No5; meldonium, parabulbar injection in a dose of 0.5 ml No 5; tiotriazolini, IM in a dose of 2.0 ml No10; group B vitamin complex, IM in a dose of 3.0 ml number 6; ascorbic acid, IM in a dose of 1.0 ml No 10; deproteinized hemoderivate of calf blood, IM in a dose of 2.0 ml No10. All patients underwent standard ophthalmic examination. Visual acuity (VA) testing was conducted using ETDRS charts (a quantity of figures). VA values ranged within 13 to 89 figures. Mean VA was 58.5 figures; mean square error 1.1. Bar graph of baseline value distribution showed that data had two maximums in areas of 20-30 and 70-80 figures. According to bimodality of VA distribution and depending on baseline VA, we subdivided groups as follows: Subgroup I of Study and Control Groups included patients with baseline VA values ? 47 figures (n=101 eyes); Subgroup II of Study and Control Groups included patients with baseline VA values > 47 figures (n=249 eyes). Study Group was divided as follows: Subgroup I, 54 eyes ? 47 fig.; Subgroup II, 133 eyes > 47 fig. Control Group: Subgroup I, 47 eyes ? 47 fig.; Subgroup II, 116 eyes > 47 fig. VA value distribution rate in groups marked is compatible with normal distribution according to Kolmogorov-Smirnov criterion (dI=0.115; р>0.20 and dII=0.096; р>0.20). There is no statistically significant difference in distribution of patients between Study and Control groups according to VA damage rates (?2=0.00008; р=0.99). Thus, in Study and Control groups were marked similar parts of patients with low (Subgroup І, 28.9% and 28.8%, respectively) and high values (Subgroup ІI, 71.1% and 71.2%, respectively). Total follow-up period was one year. Method s of statistic analyses used: Kolmogorov-Smirnov criterion was used to assess normal distribution; two-way analysis of variance (repeated measures ANOVA; factors: effect and time) followed by Newman– Keuls multiple comparison criterion; contingency tables analysis was performed using Pearson's chi-square test. Results and Discussion Baseline VA values in Subgroup I were (28.4±7.3) and (29.3±6.1) figures in Study and Control Groups, respectively (р=0.526); in Subgroup II: (71.4±9.2) and (69.5±10.2) figures in Study and Control Groups, respectively (р=0.123). At time point of 6 months after the first treatment course, VA values changed little, if at all, in Study Subgroup I, that can be considered as VA stabilization (pre-treatment (28.4±0.9) figures and post-treatment (28.6±1.0) figures, р=0.727); while in patients of Control Subgroup I was noted decrement in visual acuity from (29.3±1.0) figures to 28.2±1.1 figures (p=0.022) (Table 1).
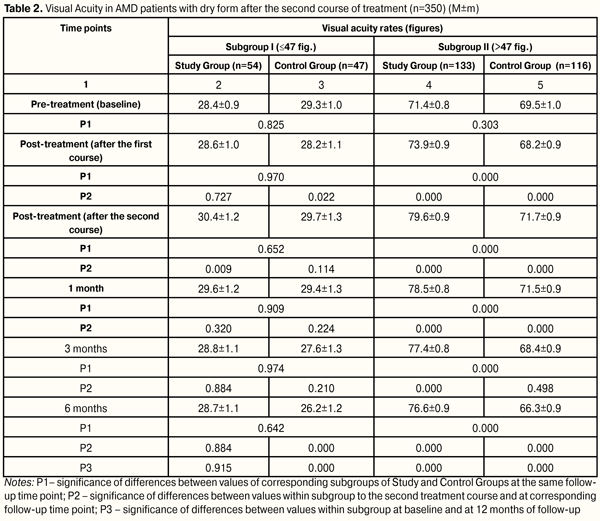
At each time point of follow-up, Study Subgroup I patients (with low vision) had advantages in distribution of number of patients with increased, stabilized and impaired VA values as compared to Control Subgroup I. At 6 months after treatment, improvement of VA values was more marked (by 24.6%) in Study Subgroup I patients than in Control Subgroup I patients, i.e. 19 eyes (35.2 %) vs. 5 eyes (10.6 %), respectively; stabilization of VA values was better by 6.0%, (17 eyes (31.5 %) vs.12 eyes (25.5 %), respectively. At the same time, decrement in visual acuity was less by 30.5% in Study Subgroup I patients than those in Control Subgroup I, i.e. 18 eyes (33.3 %) vs. 30 eyes (63.8 %), respectively. Thus, in low vision patients at 6 months after the first course, treatment success (VA improvement and stabilization) in Study and Control Groups was 66.7% and 36.0%, respectively; so, the difference in treatment success between patients undergone the first medication course in combination with LELT and those received medication only was 30.6% (11.1%?47.1%, р=0.004). At 6 months after the first treatment course, VA values increased from (71.4±0.8) to (73.9±0.9) figures, (p=0,000) in Study Subgroup II patients (with high VA rates). Meanwhile, VA rates changed for the worse in Control Subgroup II patients, i.e. from (69.5±0.9) to (68.2±0.9) figures (p=0,000). Herewith, at 6 months after the first treatment course, statistically significant difference was noted between VA rates of Study and Control Subgroups II, i.e. (73.9±0.9) and (68.2±0.9) figures, respectively, (р=0.000) (Table 1). At each time point of follow-up during the first treatment course, Study Subgroup II patients had advantages in distribution of number of patients with increased and impaired VA rates as compared to Control Subgroup II; herewith, VA stabilization was noted in a less number of patients. At 6 months after treatment, improvement of VA values was more marked, by 58.8%, in Study Subgroup II patients than in Control Subgroup II patients, i.e. 100 eyes (75.2 %) vs. 19 eyes (16.4 %), respectively; stabilization of VA values was noted in less patients, by 7.2%, i.e. (11 eyes (8.3 %) vs.18 eyes (15.5 %), respectively. Decrement in visual acuity was less by 51.6% in Study Subgroup II patients than those in Control Subgroup II, i.e. 22 eyes (16.5 %) vs. 79 eyes (68.1 %), respectively. Thus, in patients with high VA rates at 6 months after the first course, VA improvement and stabilization were noted in 83.5% of cases in Study Group patients who underwent the first course of medication and LELT treatment and in 31.9% of Control Group patients who received medication only. The difference in treatment success between patients undergone the first medication course in combination with LELT and those received medication only was 51.6% (40.1%?61.05%, р=0.000). At six months after the second treatment course, VA rate stabilization was noted in Study Subgroup I patients (pre-treatment (28.6±1.0) figures, post-treatment (28.7±1.1)) figures, р=0.496), while in Control Subgroup I there was significant decrement (pre-treatment (28.2±1.1) figures, post-treatment (26.2±1.2) figures, p=0.000). Meanwhile, VA rates increased significantly from (73.9±0.9) to (76.6±0.9) figures, (p=0.000), in Study Subgroup II patients and decreased from (68.2±0.9) to (66.3±0.9) figures, (p=0.000), in Control Subgroup II patients. Herewith, at 6 months after the second course of treatment, significant difference was noted between VA rates between corresponding Subgroups of Study and Control Groups, i.e. (76.6±0.9 and (66.3±0.9) figures, respectively, р=0.000) (Table 2).
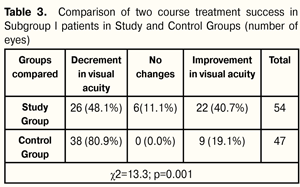
After two courses of treatment, patients of Subgroups I of Study and Control Groups had advantages in distribution of number of patients with increased, stabilized and impaired VA rates. At 12 months after treatment, VA rate improvement was by 21.6% better in Study Subgroup I patients than in Control Subgroup I ones, i.e. (22 eyes (40.7%) vs. 9 eyes (19.1%), respectively; VA rate stabilization was noted in 6 eyes (11.1% cases) of Study Subgroup I patients. Overall, treatment was more successful by 32.7% in Study Group as compared with Control Group, i.e. 28 eyes (51.8%) vs. 9 eyes (19.1%), respectively. Decrement in VA rates was less by 32.8% in Study Group than in Control Group, i.e. 26 eyes (48.1 %) vs. 38 eyes (80.9 %), respectively. (Table 3).
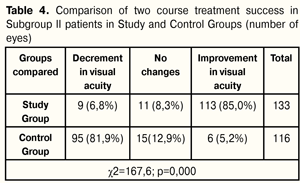
Two course treatment success in Subgroup I patients was achieved in 51.8% of cases in Study Group and in 19.1% of cases in Control Group; the difference was significant and equal to 32.7% (14.0 ? 48.15, р=0.001). After two treatment course in patients of Study and Control Subgroups II, there were noted advantages in distribution in number of patients with increased and impaired VA rates; stabilization was noted in most Control Group patients. At 12 months after the beginning of treatment, VA improvement rates were higher by 79.8% in Study Group patients than in those of Control Group, i.e. 113 eyes (85.0%) vs. 6 eyes (5.2%), respectively; VA stabilization rates were higher by 4.6% in Control Group patients than in those of Study Group, i.e. 15 eyes (12.9%) vs. 11 eyes (8.3%), respectively. Overall, treatment success was higher by 75.2% in Study Group than in Control one, i.e. 124 eyes (93.3%) vs. 21 (18.1%), respectively. VA decrement rates were less by 75.1% in Study Group patients as compared to those of Control Group, i.e. 9 eyes (6.8 %) vs. 95 eyes (81.9 %), respectively (Table 4).
Two course treatment success in Subgroup II patients was achieved in 93.3% of cases in Study Group and in 18.1% of cases in Control Group; the difference was significant and equal to 75.2% (65.4 ? 81.9, р<0.000). Monochromatic light has been shown to have a positive effect on the function of visual analyzer as well as on metabolic, hydrodynamic, and vegetative processes in the eye. It has been proved that low-energy yellow impulse light action enhances anabolic processes both in the intact retina and in experimental dystrophy [16]. Chromatic impulse photo stimulation is used for critical flicker frequency (CFF) diagnostic method, and for treatment of retina and optic nerve pathology [11]. Light therapy has been proved to be effective in complicated high myopia and partial optic nerve atrophy [8, 9]. Photo stimulation using red impulse light is effective in amblyopia treatment [2]. Green monochromic light has been shown to have a positive effect on the eye hydrodynamics [17]. However, previous papers have used monochromic light or two wave length combination. We used modulated impulses of three wave lengths: green, red, and infra-red spectra which are more tropical for certain structures of the eye. We believe that the positive functional effect of low-energy light stimulation could be achieved not only through wave length selected but pulse modulation, i.e the ratio of pulse’s length and frequency, energy, and radiation power density. We suppose that low-energy monochromic light of green, red, and infra-red spectra plays the part of a stimulus which enables to take the retina to the next energetic level, to renew regulatory mechanisms of redox process compensation in the macula, to improve retinal and choroidal cell resistance to damaging factors, to renew the balance in vegetative nervous system, to run reactions both at molecular level and in the whole organism. Low-energy light therapy increases optic density of macular pigment and stabilizes morphological structure of the macula in the dry form of AMD, which has been proved in our previous papers. Thus, VA testing data have shown that law-energy light therapy in addition to medication enables to stabilize and to maintain visual acuity in patients with dry form AMD by contrast with medication only therapy. Conclusions 1.It was found that AMD patients with low vision had stabilization of VA rates (pre-treatment (28.6±1.0) figures, post-treatment (28.7±1.1) figures, р=0.496) after two joint courses of low-energy light therapy and medication; meanwhile, patients receiving medication only therapy had significant decrement in visual acuity from (29.3±1.0) to (26.2±1.2) figures (р=0.000). 2.It was revealed that, after two joint courses of low-energy light therapy and medication, AMD patients with high VA rates had significant increase of VA rates from (71.4±0.8) figures at baseline to (76.6±0.9) figures at 12 months, (р=0.000); while VA rates decreased from (69.5±0.9) to (66.3±0.9) (р=0.000), respectively, in patients receiving medication only therapy. 3.It was noted that treatment of AMG patients both with poor and high VA rates who underwent two joint courses of low-energy light therapy and medication was more successful than in those who received two courses of medication only therapy. The difference between them was 32.7% (14.0 ? 48.15. р=0.001) for poor VA rates and 75.2% (65.4 ? 81.9, р<0.000) for high VA rates. 4.Success of low-energy light therapy in combination with medication course in AMD patients depends on baseline VA rates: the higher visual acuity is, the more successful is the treatment. References 1.Bibkov MM, Faizrakhmanov RR, Yarmukhametova. [Age-related macular degeneration]. M.: Aprel; 2013. 196p. Russian. 2.Venger LV. [Efficacy of potostimulation with monochromatic pulsed light in restorative treatment of amblyopia patients]. Odeskii Med. Zhurnal. 2001;3:82-6. Ukrainian. 3.Yevsyukova O. [Local immunity changes in patients with dry form of age-related macular degeneration]. Oftalmologiia. Vostochnaia Evropa. 2014;2(21):41-7. Russian. 4.Egorov EA, Romanenko IA. [Age – related macular degeneration]. KOFT. 2009;1:42-5. Russian. 5.Karliychuk M. [Role of combined nutraceuticals in the prevention of development and progression of age-related macular degeneration]. Oftalmologiia. Vostochnaia Evropa. 2014;2(21):139-49. Russian. 6.Kryzhanovska TV. [Disability due to eye pathology in the population of Ukraine in the years 90-2002]. Oftalmol Zh. 2003;3:23-7. Ukrainian. 7.Kryzhanovska TV. [State and topical issues of prevention of blindness and hypovision in Ukraine]. Oftalmol Zh. 2002;6:67-70. Ukrainian. 8.Kuliakin MI, Parame? VT, Kliutsevaia EI, Savostenko IG. [Phototherapy of high complicated myopia]. Oftalmol Zh. 1981;36(4):228-31. Russian. 9.Kuliakin MI, Kliutsevaia EI, Parame? VT, Savostenko IG. [Phototherapy of partial optic atrophy]. Oftalmol Zh. 1982;37(3):159-62. Russian. 10.Logai IM, Sergienko NM, Kryzhanovska TV. [Blindness and hypovision in Ukraine and current issues of their prevention.] X Congress of Ophthalmologists of Ukraine: Thesis. 28-30 May 2002. Odessa: 2002. 10-11. Russian. 11.Marchenkova TE, Mironova EM, Golubtsov KV, Arnoldova AV. [Application of chromatic pulse photostimulation for treatment of retinal and optic nerve pathology]. Oftalmol Zh. 2006;3:27-30. Russian. 12.Nagorna AM, Rykov SO, Varyvonchyk DV. [State of eye morbidity of population in Ukraine]. Ophtalmol Zh. 2003; 3:28-33. Ukrainian. 13.Pasechnikova NV, Korol AR, Zadorozhny OE, Kustrin TB, Nasinnik IO. [Modern principles of diagnosis and treatment of age-related macular degeneration]. Oftalmol Zh. 2013;4:93-107. Ukrainian. 14.Petrunya A. M., Yevsyukova O. A. [Influence of immunotropic drugs on humoral immunity in patients with a dry form of age-related macular degeneration]. Oftalmol Zh. 2013;2:43-7. Russian. 15.Scrypnyk R, Scripnichenko I. [Prophylactics and treatment of age-related macular degeneration]. Oftalmologiia. Vostochnaia Evropa. 2014;1(20):100-3. Russian. 16.Soldatova AM. [The role of free radical oxidation-reduction processes and visible light in the pathogenesis of sclerotic macular degeneration and its differentiated treatment]. Author’s thesis for Dr. Of Med. Sc.: 14.00.18. Filatov Institute of Eye Diseases and Tissue Therapy. Odessa; 1992. 36p. Russian. 17.Shargorodska IV. [Effect of monochromatic light on hydrodynamics of the eye]. Author’s thesis for Cand. Of Med. Sc.: 14.00.18. Shupyk National Medical Academyof Postgraduate Education. K.; 2003. 19p. Ukrainian. 18.Camparini M, Cassinari P, Ferrigno L. et al. ETDRS–Fast: Implementing Psychophysical Adaptive Methods to Standardized Visual Acuity Measurement with ETDRS Chart. Inv. Ophthalmol. Vis. Sc. 2001; May 42(6):1226–31. 19.Vanden Bosh ME, Wall M. Visual acuity scored by the letter-by-letter or probit methods has lower retest variability then the line assignment method. Eye. 1997;11(3):411–7.
|