J.ophthalmol.(Ukraine).2016;5:35-40.
|
https://doi.org/10.31288/oftalmolzh201653540 Clinical characteristics of patients with choroidal melanoma of small sizes I.V. Tsukanova, Junior Researcher Associate Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine Odessa, Ukraine E-mail: inna.sister@mail.ru Introduction. Coroidal melanomas (CM) of small sizes, that include CM with height up to 3.0-4.0 mm, comprise 5-21% among all melanomas. According to international TNM classification (2009), T1 CM stage includes melanomas protruding into the vitreous up to 3.0 mm and having the base length up to 12.0 mm and melanomas protruding from 3.1 to 6.0 mm and having the base length up to 9.0 mm. Thus, CMs of small sizes (T I stage) refer to melanoma protruding up to 3.0 mm and having the base length up to 12.0 mm Since CM can be early diagnosed as result of active improvement of diagnostic methods, there is a need of an extended use of organ-sparing treatment modes, in which successful local result, so called local control, and favorable life prognosis for patients can be expected. In view of this, close investigation of different tumor characteristics which can help in choosing adequate treatment approach and in predicting its endpoint is of a great necessity and relevance. The purpose of the present paper was to study clinical characteristics of T1 CM stage (protruding to the vitreous up to 3.0 mm and the base length up to 12.0 mm) in patients treated at State Institition “The Filatov Institute of Eye Diseases of Tissue Therapy of NAMS of Ukraine” Material and Methods. We studied case histories of 88 patients with CM of small sizes received medical treatment at SI “The Filatov Institute of Eye Diseases of Tissue Therapy of NAMS of Ukraine”within 2004 and 2016. All patients underwent standard ophthalmological and general examination Сine-Scan ultrasound sonography, liver ultrasound test, and lung fluorography/roentgenography. At the moment of referral, none of patients were revealed metastasis signs. Statistical data were processed using “Statistic 9.0” software. Mean values are given as М(SD). Results. T 1 CM stage was observed almost 2.5 times more often in women (71.6%) as compared to men affecting working-age persons, average age 55.9 (12.8) y/o. At baseline of treatment at SI “Filatov Institute of Eye Diseases and Tissue Therapy”, CMs of small sizes were mainly of paracentral and juxtapapillary lacation, 37 (42.05%) and 26 (29.55%) patients, respectively; tumor protruding averaged 1.89(0.73) mm and mean tumor base length was 7.36 (2.2) mm. In 86 patients (96.74%) the tumor base length exceeded 3 mm. Unevenly pigmented tumors were observed rarely, only in 5 patients (5.68%). The tumor borders were indistinct in most cases (88.64%). Conclusions. Clinical characteristics of T1 CM stage were studied at baseline on dependence of sex, lacation, size, pigmentation, and tumor growth. Key words: T1 stage choroidal melanoma, choroidal melanoma of small sizes, clinical characteristics
Introduction Uveal Melanoma (UM) is a severe eye disease which leads not only to a loss of vision functions and even of the eye as an organ itself but also to metastatic disease resulting in the death of a patient. Despite the fact that in the overall population choroidal melanoma (CM) is comparatively rare and diagnosed in 2-13.3 persons per 1 000 000 of adults [2, 7], its ratio among all intraocular tumors is 72-85% [4, 7, 8]. Comprising 5- 21% among all the CM [5, 6, 8, 16, 25], a special place belongs to CM of small sizes, that include CM with height up to 3.0-4.0 mm [22, 28, 30]. According to World Health Organization international classification of tumors designed in 2009 by American Joint Committee on Cancer (AJCC) and L’Union Internationale Contre le Cancer (UICC) on the basis of processing the sixth edition of TNM classification of humors in 2002, T1 MC stage includes melanomas protruding into the vitreous up to 3.0 mm and having the base length up to 12.0 mm and melanomas protruding from 3.1 to 6.0 mm and having the base length up to 9.0 mm [3]. Thus, T1 CM as a primary disease stage is divided into two sub-stages, in one of which, small size CM refers to melanoma protruding up to 3.0 mm and having the base length up to 12.0 mm, which we shall follow in our research. Since CM can be early diagnosed as result of active improvement of diagnostic methods, there is a need of an extended use of organ-sparing treatment modes, in which successful local result, so called local control, and favorable life prognosis for patients can be expected. In the meantime, adequate and early treatment conditions its high efficacy in regard to both preserving the visual functions of the eye and better prognosis for patient’s life quality [5, 9, 10, 11, 16, 22, 27, 29, 30, 32]. In view of this, close investigation of different tumor characteristics which can help in choosing adequate treatment approach and in predicting its endpoint is of a great necessity and relevance. The purpose of the present paper was to study clinical characteristics of T1 CM stage (protruding to the vitreous up to 3.0 mm and the base length up to 12.0 mm) in patients treated at State Institition “The Filatov Institute of Eye Diseases of Tissue Therapy of NAMS of Ukraine” Material and Methods 88 patients with CM of small sizes received medical treatment within 2004 and 2016. All patients underwent standard ophthalmological examination including visometry, tonometry, refractionometry, visual field test, campimetry, biomicroscopy, Сine-Scan ultrasound sonography, optical coherence tomography (OCT) using Stratus OCT model 3000 (Karl Zeis), and fluorescent angiography. Also, all patients underwent a general clinical examination, regional lymph node condition was assessed by its palpation, and the condition of the liver and lungs was determined according to the data of ultrasound test and fluorography/roentgenography, respectively. At the moment of referral, none of patients were revealed metastasis signs. To perform statistic analysis, e-database was designed, in which parameters of all tests were recorded as either absolute counts or category indication in qualitative characteristics of the parameter. Statistical data were processed was made “Statistic 9.0” software. Mean values are given as М(SD). Results and Discussion The average age of the patients with CM of small sizes was (55.9±12.8), the minimal and maximal ages were 23 and 82, respectively; this coincide with data that CM develops active working age persons within 50.9-62.5 y/o [13, 16, 20, 26]. There were 63 (71.6%) women and 25 (28.4%) men. The right eye was affected in 41 patients (46.6%) and the left eye was affected in 48 patients (53.4%). So, women prevailed almost three times in patient cohort. The most frequently, tumor developed in working-age persons. No significant difference was revealed in between tumor incidence in the right and left eyes. Before admission to the Filatov Institute, 12 patients (13.6%) treated at the place of residence, of them, 5 patients were treated due to choroidoretinitis, 1 to proliferative chorioretinopathy, 1 to subretinal haemorrhaging, 2 to retinal degeneration, 2 to nevus, and, in 1 patient, tumor was revealed during cataract extraction. 76 patients (86.4%) applied for primary diagnostics and treatment to the Filatov Institute. Main complaints of patients were reduced vision acuity in 78 patients (88.64 % of cases), flashes of light or “lightning” in the eye in three patients (3.41%), dark spot in front of the eye in two patients (2.27%), blurry vision in one patient (1.14%), and there were no complaints in four patients (4.55%), in who the tumors were revealed accidentally. To assess the changes in visual acuity (VA) in patients, we used such categories of VA as follows: 0 ? vision loss; 1 ? light perception to 0.1; 2 ? 0.12 to 0.25; 3 ? 0.3 to 0.6; 4 – 0.7 to 1.0. Distribution of patients according to VA category is given in Table 1.
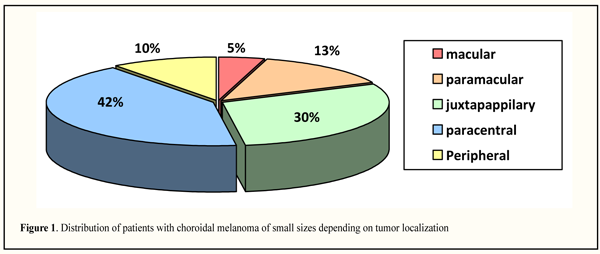
As it can be seen in the Table 1, at the baseline, blindness was in two patients (2.27%), VA was defined as high, from 0.7 to 1.0, in 13 patients (14.77 %), in six of them VA was 1.0. The patients were distributed almost evenly in categories 1, 2, and 3. Our data on visual acuity are quite different from the literature data, according to which most patients with CM of small sizes at the moment of tumor detected had high VA, equaling 0.5-1.0 in 80% and 0.1 and lower only in 6.2% of cases [12, 25, 28, 33]. On dependence on ocular refraction, patients were distributed as follows: emmetropia, 22 patients (25.0%); myopia, 12 patients (13.64%); hyperopia, 54 patients (61.36%), meaning that the most patients had hyperopia. On admission, transparent cornea was noted in all patients; lens opacity of different degree was revealed in 50 patients (56.82%); pseudophakia was in 6 patients (6.82%); various degree opacities of the vitreous were noted in 60 patients (68.2%), one of them had partial intraocular hemorrhage. It should be noted that intraocular pressure was within norm and ranged from 17 to 19 mm Hg. Tumor localization patterns on the fundus are presented in Figure 1. Moreover, we divided tumors according to their location site as follows: macular, when tumor spread on the fovea; paramacular, when tumor edged to the fovea without spreading in it; juxtapapillary, when tumor bordered intimately to the optic nerve regardless of touch area or was 1 mm distanced from it; paracentral, when one tumor edge was 3 mm side of the fovea and peripheral edge did not reach the equator; peripheral, when tumor placed pre-equatorially. We accepted such distribution, firstly, due to anatomy of the eye; secondly, in view of specific importance of primary location of the tumor for functional prognosis; and, thirdly, due to features of technical approach to treatment of differently-located tumors.
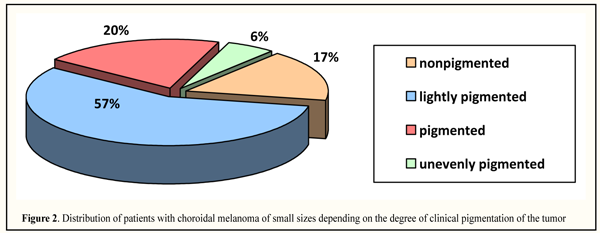
Paracentral location was observed more often, in 37 patients (42.05%), followed by juxrapappilary and paramacular locations, 26 (29.55%) and 12 (13.64%), respectively; less frequently, small size tumors were revealed in the periphery and in the macular area, 9 (10.23%) and 4 (4.55%), rtespectively. In paracentral localization, tumors were located in the outer part, in 24 patients (64.86%), including 14 patients (58.33%) with upper-outer quadrant location. It should be noted that tumor location in the macular area and in the extreme periphery was observed equally as often in both right and left eyes; paramacular location was more often in the right eye (in 9/12 eyes, 75.0%) while juxtapapillary and paracentral locations were more often in left eyes, 16/26 (61.54%) and 22/37 (59.46%) eyes, respectively. According to literature data, CM of small sizes are located more frequently in the central part of the fundus, in macular area and juxtapapillarly, 26-42% and 31-50%, respectively [25, 28, 30, 32]. The degree of clinical pigmentation of CM was defined as follows: nonpigmented (amelanotic), lightly pigmented, pigmented and unevenly pigmented. Distribution of patients with CM of small sizes depending on the clinical pigmentation of the tumor is presented in Figure 2 indicating that lightly pigmented tumors were noted more frequently, i.e. in 50 patients (56.82%); pigmented and nonpigmented tumors were observed in 18 (20.45%) and 15 (17.05%) patients, respectively; unevenly pigmented tumors were very rare, only in 5 (5.68%) patients.
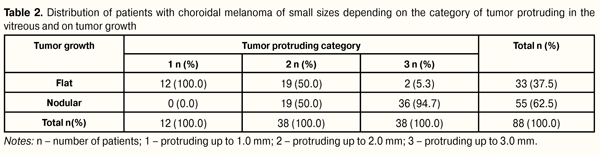
Despite the literature data which have pointed the prevalence of pigmented tumors, among our patients prevailed lightly pigmented T1 tumors which have a better prognosis since the percentage of mixed and epithelioid cell histological types is lower among light tumors [1, 14, 21, 22]. CMs of small size were of flat and nodular growths in 33 (37.5%) and in 55 (62.5%) patients, respectively. The tumor borders were indistinct in most cases, i.e. in 78 patients (88.64%). Secondary retinal detachment was revealed significantly more frequently in nodular-grown tumors (in 26 patients, 29.55%) and was located under and above the tumor in 84.62% and 15.38% of cases, respectively (р = 0.0005). Tumor height was assessed sonographically and averaged 1.87 (0.68) mm with 0.3 mm as minimal and 3.0 mm as maximal values. To assess changes in the follow-up period after treatment, the patients were divided into 5 categories according to tumor protruding. Distribution of patients depending on the tumor protruding in the vitreous and on tumor form is given in Table 2.
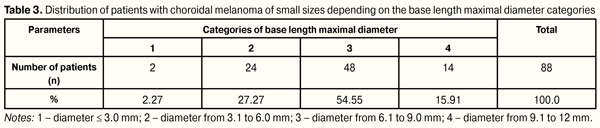
Data presented in Table 2 show that, in our T1 CM stage patients, more frequent tumor protruding was up to 2.0 mm and from 2.0 to 3.0 mm, in 50 (56.82%) and 38 (43.18%) patients, respectively. It can be noted that significant protruding from 1.0 to 2.0 mm was defined equally as often both in flat and nodular tumor growth, whilst that from above 2.0 mm was common only for nodular growth of tumor (?2=39.4, р = 0.00000). Analyzing tumor protruding depending on the eye affected, we revealed that up to 1.0 mm protruding was more often observed in the right eye, in 11/12 patients (91.67%); in the left eye, tumor protruding was almost twice as often noted both up to 2 mm (63.16%) and up to 3 mm (57.89%) (?2=11.56, р=0.003). Base length of T1 stage CM was assessed sonographically in two directions, along its minimal and maximal diameter. Minimal diameter length averaged 6.72 (1.89) mm with minimal and maximal values 2.5 mm and 11.0 mm, respectively; maximal diameter length averaged 7.41 (2.09) mm with minimal and maximal values 2.5 mm and 12.0 mm, respectively. To assess follow-up changes in tumor length we analyzed parameters of maximal diameter base length according to TNM classification. In this regard, the patients were divided into categories as follows: 1, diameter ? 3.0 mm; 2, diameter from 3.1 to 6 0 mm; 3, diameter from 6.1 to 9.0 mm; 4, diameter from 9.1 to 12 mm, which was corresponding to TNM classification. Distribution of T1 stage CM patients depending on the base length maximal diameter values is given in Table 3.
Table 3 data show that most patients had tumor base length diameter > 3.0 mm, 86 patients (97.73%); herewith, 48 patients (55.81%) had tumor base length diameter from 6.1 to 9.0 mm. According to the literature data, initial size of the tumor predetermines largely treatment and disease outcomes. Within 5 year follow-up period, metastases develop after small size CM treatment in 3-16% of cases vs. 23-53% after treatment of CM of medium-sized and large CM [14, 19, 22, 27, 30]. Among metastasis risk factors are tumor growth in height and length [15, 17, 23, 24] as well as its juxtapapillary location and blurred vision symptoms [27]. Thus, Shields C.L. et al., while studying CM of small sizes, have revealed that tumor growth 8 times increases the risk for metastases (as compared to patients without metastasis), and, in the presence of protruding more than 1.1 mm in combination with juxtapapillary location, it increases 81 times [27]. Conclusion Thus, our study showed that T 1 CM stage was observed almost 3 times more often in women (71.7%) as compared to men, affecting working-age persons, average age 55.9(12.8) y/o. At baseline of treatment at SI “Filatov Institute of Eye Diseases and Tissue Therapy”, CM of small sizes were mainly of paracentral and juxtapapillary lacation, 37 (42.05%) and 26 (29.55%) patients, respectively; tumor protruding averaged 1.89(0.73) mm and mean tumor base length was 7.36 (2.2) mm. In 86 patients (96.74%) the tumor base length exceeded 3 mm. Lightly pigmented tumors were observed in 50 (56.82%) patients, which was more frequently than pigmented and nonpigmented ones, in 18 (21.74%) and 15 (17.05%) patients, respectively. Unevenly pigmented tumors were observed rarely, only in 5 patients (5.68%). The tumor borders were indistinct in most cases (88.64%).
All patients underwent organ-sparing treatment using transpupillar diode laser thermotherapy mode developed by us; the treatment outcomes will be reported in the further papers. References 1.Vit VV. [Radiant pathomorphosis of malignant melanomas of the uveal tract of the human eye]. Oftalmol Zh. 1989;6:321-5. Russian. 2.Ioilev EN, Fradkina IA. [Analysis of malignant tumors of the eyeball]. [Tumors and tumor-like ophthalmological diseases: proceedings]. M.;1998. 28-31. Russian. 3.Classification of eye choroidal melanomas. Translated by Buiko AS. Oftalmol Zh. 2010;6:20-30. Russian. 4.Panova IE, Pozdeeva OG, Semenova LE et al. [Laser surgery in the combined treatment of intraocular melanomas]. [New laser technologies in ophthalmology: Proceedings]. Kaluga; 2002. 109. Russian. 5.Libman ES, Brovkina AF, Bezrukov AV. [Afterhistory of uveal melanoma. Comparative assessment of enucleation and organ-sparing treatment modes]. Oftalmol Zh. 1989;6:336-8. Russian. 6.Linnik LF, Magaramov DA, Yarovoi AA, Semikova TS. [Laser transpupillar thermotherapy of choroidal melanoma]. Oftalmokhirurgiia. 2002;3:45-50. Russian. 7.Brovkina AF, Valskii VV, Gusev GA et al. [Ophthalmic oncology]. Brovkina AF, the Editor. M.:Meditsina; 2002. 424 p. Russian. 8.Semikova TS, Yarovoi AA. Efficacy of combined treatment of choroid melanoma. VII Congress of Ophthalmologists of Russia. Proceedings, Part II. M.; 2000: 124. Russian. 9.BartlemaYM, Keunen JEE, Oosterhuis JA, Journee-de Korver JG. Five year follow-up of 50 patients with choroidal melanoma after combined treatment with brachytherapy and transpupillary thermotherapy. Xth International congress of ocular oncology: Final programme and abstract book. Amsterdam, the Netherlands. 2001: 176. 10.Biswas J, Kabra S, Subramanian K, Shanmugam M. Clinical and histopathological characteristics of uveal melanoma in asian Indians: a study of 117 patients. XI International congress of ocular oncology: programme and abstract book. Hyderabad, India. 2004: 99. 11.Collaborative ocular melanoma study (COMS) randomized trial of I - 125 brachytherapy for medium choroidal melanoma: I. Visual acuti after 3 years, COMS report no. 16. Ophthalmology. 2001;108:348-66. 12.Currie ZI, Rennie IG, Talbot JF. Retinal vascular changes associated with transpupillary thermotherapy for choroidal melanomas. Retina. 2002; 20(6):620-6. 13.Augsburger JJ, Correa ZM, Schneider S et al. Diagnostic transvitreal fine - needle aspiration biopsy of small melanocyte choroidal tumors in nevus versus melanoma category. Trans. Am. Ophthalmol. Soc. 2002;100:225-32. 14.Diener-West M, Hawkins BS, Markowitz JA, Schachat AP. A review of mortality from choroidal melanoma. II. A meta - analysis of 5 - year mortality rates following enucleation, 1966 through 1988. Ophthalmol. 1992;110:245-50. 15.Shields CL, Shields JA, Karlsson U et al. Enucleation after plaque radiotherapy for posterior uveal melanoma: histopathologic findings. Ophthalmology. 1990;97(12):1665-70. 16.Saornil MA, Ordonez JL, Almaraz A et al. Epidemiologic profile of uveal melanoma patients in Spain. Xth International congress of ocular oncology: Final programme and abstract book. Amsterdam, the Netherlands. 2001: 293. 17.Gunduz K, Shields CL, Shields JA, Cater JR. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am. J. Ophthalmol. 1999;127(5):579-89. 18.De Potter P, Shields CL, Shields JA et al. Impact of enucleation versus plaque radiotherapy management of juxtapapillary choroidal melanoma on patient surviva. Br. J. Ophthalmol. 1994;78:109-14. 19.Kaiserman I. Forecasting the prognosis of uveal melanoma using an artificial neural network. XI International congress of ocular oncology: programme and abstract book. Hyderabad, India. 2004: 65. 20.Kociecki J, Pecold K, Wieckowska A. Results of treatment of intraocular malignant melanoma with diode laser transpupillary thermotherapy. Xth International congress of ocular oncology: Final programme and abstract book. Amsterdam, the Netherlands. 2001: 301. 21.Lee DS, Anderson SF, Perez EM, Townsend JC. Amelanotic choroidal nevus and melanoma: cytology, tumor size and pigmentation as prognostic indicators. Optom. Vis. Sci. 2001;78(7):483-91. 22.Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch. Ophthalmol. 1997; 115(7): 886-93. 23.De Potter P, Shields CL, Shields JA et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: visual acuity and survival outcome. Arch. Ophthalmol. 1996;114:1357-65. 24.Augsburger JJ, Gamel JW, Shields JA et al. Post - irradiation regression of choroidal melanomas as a risk factor for death from metastatic disease. Ophthalmology. 1987;94(9):1173-7. 25.Shields CL, Shields JA, Peres N et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109(2):225-34. 26.Primavera V, Russo V, Laculli C, Delle N. Transpupillary thermotherapy for small choroidal melanoma: results in 25 patients. Xth International congress of ocular oncology: Final programme and abstract book. Amsterdam, the Netherlands. 2001: 292. 27.Shields CL, Shields JA, Kiratli H et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102(9):1351-61. 28.Robertson DM, Buettner H, Bennett SR. Transpupillary thermotherapy as primary treatment for small choroidal melanomas. Arch. Ophthalmol. 1999;117:1512-9. 29.Seregard S. Long - term survival after ruthenium plaque radiotherapy for uveal melanoma. A meta - analysis of studies including 1066 patients. Acta. Ophthalmol. Scand. 1999;77(4):414-7. 30.Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr. Opin. Ophthalmol. 2002;13(3):135-41. 31.Singh AD, Eagle RC, Shields CL, Shields JA. Enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch. Ophthalmol. 2003;121:397-400. 32.Sambuelli R, Luna JD, Reviglio VE et al. Small choroidal melanoma with massive extraocular extension: invasion through posterior scleral emissary channels. Int. Ophthalmol. 2001;24(4):213-8. 33.Shields CL, Shields JA, Kiratli H et al. Transpupillary thermotherapy choroidal melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998;105(4):581-90.
|