J.ophthalmol.(Ukraine).2017;4:60-73.
|
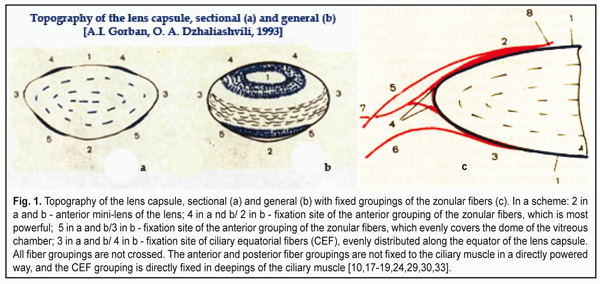
https://doi.org/10.31288/oftalmolzh201746073 Optical features of light passing through refractive structures of the eye I.N. Koshits1, director, O.V. Svetlova2, Dr. Sc. (Med.), Prof., M.G. Guseva3, ophthalmologist, D.V.Pevko4, executive editor, M. B. Egemberdiev5, Cand. Sc. (Med.) 1 Petercom-Networks / Management Systems Consulting Group Cl. corp., St. Petersburg, Russia; 2 The I.I. Mechnikov Northwestern State Medical University, St. Petersburg, Russia; 3 Diagnostic municipal Center «Vodokanal Spb.», St. Petersburg, Russia; 4 “Glaz” journal, Moscow; 5 United Hospital of the «Chuya» District, Department of Ophthalmology, Bishkek, Kyrgyzstan The features of light refraction in the anterior segment of the eye and after dispersion on the posterior surface of the cornea are considered. It is shown that the cornea refracts the light as a weakly scattering lens. The conclusion is made that the optical system of the eye is essentially a kind of natural telescope. The main task of the anterior segment of the eye is the optical compression of the light coming from the surrounding into a beam (tunnel) of light 1.8–2.5 mm in diameter, which can pass to the foveola even through a narrow pupil. The concept of «a light tunnel» was introduced. The task of the eye lens is the effective optical control of the three basic dispersion bands of blue, green and red colors (RGB-bands) in «the light tunnel». The optical control is carried out with the two mini-lenses located in the center of the anterior and posterior surfaces of the lens capsule. Perhaps, the main mechanism of lens accommodation is the changes in the curvature of these mini-lenses when looking closer or farther away. The executive mechanism of «tunneling» accommodation is the varying pressure inside the lens, which is maximal when viewed near, when the elastic capsule of the lens is slightly stretched, and it can most strongly compress the lens masses. Taking into account the physiological makers of the ways of delivery and removal of aqueous humor, which is necessary to maintain the metabolism of the internal structures of the lens, a practical conclusion is made about the possible rate of cataract development in hypermetropic and myopic patients when optical correction is prescribed improperly. Recommendations for rational optical correction are given. Key-words: optics of the eye, light tunnel, accommodation, mini-lenses of the lens, cataract Introduction Traditional concepts of the optical system of the human eye is currently accepted and even included in school textbooks. It is generally thought that the optic tract of the eye has completely been studied and requires no serious investigations. However, there are still a lot of “awkward” questions which still have no answers so far. In particular, the following issues are not completely clear: • How do lateral rays of the light get to the periphery of the lens and get refracted rather that blocked by the iris in case when the pupil is narrow? • How can light rays be refracted when they are near the optic axis of the lens, where the curvature of the anterior and posterior surface of the lens capsule can be relatively small compared to the periphery? • Where does the white light get dispersed: inside the cornea or at its posterior surface? • What kind of optical signal is one coming to the retina: are these circles or bands of light scattering of red, green, and blue colors (RGB-bands) that can be detected by the rods in the macula? • How does a system of “binocular focusing” on the object examined work in both eyes and how does their individual optical sight work? • What contribution does the cornea, which is a divergence lens, make to a “converging” optic system of the anterior segment of the eye? • What must be considered as a focus of the eye since it is, in fact, impossible to simultaneously combine the foci of three RGB- wavefronts on any common focal plane because of the presence of optic axial aberration? • What highly-reliable optical signal must be formed by accommodative mechanisms in the macula for the brain to be able to “detect” the moment of accurate eye’s focusing on the separated spaces or to set a sharp focus on the object? • What parameters of the RGB-bands, formed by the optical system of the eye, must the macula itself and the fovea, in particular, be able to fix for a feedback signal to be formed in the brain for controlling the ciliary muscle and accommodation system in whole? • In what way, for instance, do nightwear orthokeratology lenses change the topography and refractive power of the cornea? “Optical switching off” of which physiological adaptive mechanisms makes ortho-k lenses most successful in controlling acquired mypia? Even a superficial glance at the above listed questions makes it possible to conclude, which is disappointing, that “something is not so good in the optical ocular state” and that optometry, so far, is a poorly developed area of knowledge. This is so because there can be much more such “awkward questions” than it’s been given. And something should be done in this regard despite the fact that optometric beliefs often hinder onward progress. The first brightest example of this is the absence, up until now, of a generally-accepted theory of accommodation. Although a theory of lens accommodation by genial Helmholtz has stood the test of time and, essentially, does not go against the laws of mechanics, it can be considered only as a first step in understanding how accommodation acts in whole. It should be noted that, up to date, there have been found a great number of additional accommodative mechanism in the eye and even their classification has been developed [14, 17-19, 24, 26, 29, 30, 33, 38-43]. Besides, there have been completed the first hypothetical ideas on the revealed new mechanisms which provide a sharp image through forming such a signal in the macula that is adequate to macula’s physiological capability [12, 13]. However, a lot of open questions in the eye optics, to our opinion, are the main brake today in creating a new theory of accommodation, adequate to the lows of optics and physics. Another bright example is the long-term absence of an efficient theory of myopia. Incremental retinal-defocus theory (IRTD), which is rather popular today, is contradictory and its hypotheses often have no distinct morphological and physiological grounds [21, 25]. Since acquired myopia is traditionally considered as a disease rather than a normal adaptive process of adjusting the axial length to the decreased visual loads in the conditions of “display” civilization, it is much more difficult to search for real mechanisms of axial length elongation. A metabolic theory of adaptive myopia, presented by I.N. Koshits and O.V. Svetlova in 2001, has eliminated partially this gap and has already passed successfully clinical approval with follow-up periods of 3, 5, and 7 years [8, 9, 13, 31]. It has also become clear that, in the way of creating more effective solutions for optical corrections, there is a lack of understanding the main features of how the optical part of the visual tract of the eye, including the electrical one, functions [1]. Taking into account the fact that the humanity expects the most massive epidemic in its history, which is “a pandemic of short-sightedness” and when more than a half of the Earth population (5.5 billion) will be myopes by 2050 [13,20,37], deep investigations of the ocular optics become especially relevant. It should also be noted that a weak study of theoretical bases of optometry has made many ophthalmology practitioners go with their gut feelings when choosing optical correction because of the clear inconsistency of numerous “guidelines”. This is so because, starting with 1960s, our optometrists were recommended for myopes to use mainly full bearable correction for distance [6]; afterwards, there was a massive turn to incomplete correction for distance by the end of the 20th century [2-5]. This is not so long ago that we understood that what is needed is rational optical correction enabling to use mild over-correction for near and distance. And in case of long hard work with displays, it is needed to use for near full correction or mild under-correction with consideration of possible fatigue of the ciliary muscle [8, 9, 31]. If you take a detailed look at features of gradual changes in a physiological effect of nightwear orthokeratology lenses on the eye’s refraction during the working day, it will be clear that this is our strategy that they realize! Later on, all this will be addressed in more details. Rational correction is required to maintain a certain tone of the ciliary muscle and to provide, by means of the aqueous humor, transmitting through the uveascleral pathway a sufficient quantity of ingredients for healthy metabolism of collagen structures in the intermediate and posterior parts of the sclera in order to prevent its anterior pole from elongation [11, 15, 16, 19, 23, 26-28]. All above made it draw the focus on the eye’s optics as the first chain in the accommodation control system. And this is one of the reasons for the present paper to appear as a logical extension of three preceding ones [12, 13, 21]. 1. Structure of the lens capsule and its interaction with zonular fibers In 1993, A.I. Gorban and O.A. Dzhaliashvili, outstanding Russian ophthalmologists, published an astonishing book with A.I. Gorban’s own drawings which did not contain a single mistake from the viewpoint of the mechanics laws [7]. And today, this book has not lost its edge since it was decades ahead of time even that time. Some authors of this paper have had the honor to personally discuss the issues of biomechanics of the lens and accommodative mechanisms with A.I. Gorban and O.A. Dzhaliashvili and can testify to their understanding not only the laws of mechanics but also other natural laws including optics. They both had deep encyclopedic knowledge. This was the natural cross-disciplinary feel that had helped them create such an educational masterpiece. Figure 1 demonstrates their and our vision of a general composition of the lens bag with the sites of fixation of zonular fibers. Hard circular thickening of the lens capsule can be seen in the sites of fixation of anterior and posterior groupings of the zonular fibers. This is, in fact, a kind of thick circumferential collagen ligament, the ring of which can hardly be stretched in all sides.
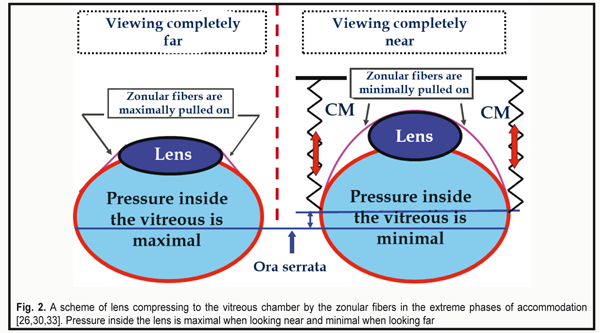
As for a grouping of so-called “bridle” equatorial fibers (Fig.1B, 4), it is connected to a thinner equatorial part of the lens and helps keep the lens centrally to the optic axis of the lens in any position of the head and even in zero gravity [30, 33]. In all phases of accommodation, the lens is pushed by the forceful anterior grouping of the fibers to the vitreous chamber. The zonular fibers (ZF) are maximally pulled on when viewing far objects and slacken (not relaxed) when viewing near ones. This makes the lens capsule become maximally rounded and to more forcefully compress the lens fibers (Fig. 2) [26, 30, 33]. The lens pushing on to the vitreous body by the zonular fibers and the Wieger's ligament occurs in all accommodation phases and allows such damping mechanism not only to securely keep the lens in the eye but also to damp its oscillations at the inertial load.
A generalized drawing of collagen structures of the lens is given in the educational course of American Academy of Ophthalmology (Fig. 3). It is well seen how forceful the anterior and posterior groupings of the fibers are in relation to the lens capsule structures. Prof. V.V. Volkov has called them “reins”, which definitely characterizes their biomechanical essence. It should be also noted that along the lens equator inside the lens there are elastic fibers (left bottom part of the figure) which help to round the lens when the tension of zonular fibers are slackened.
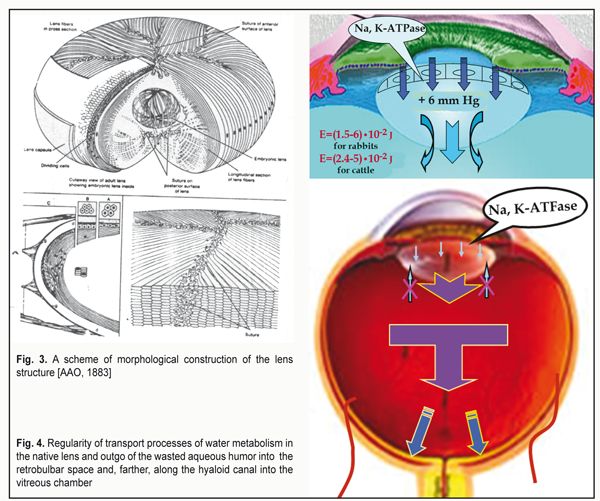
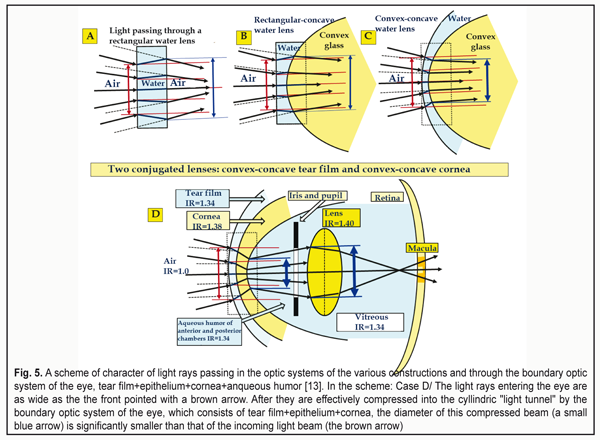
Here, it should be noted that the lens is provided by nutrition by the aqueous humor from the posterior chamber with the help of the lens epithelium located on its anterior portion. Modern investigations of the paths and conditions of the lens nutrition which have been performed by a biophysicist in numerous native lenses of different animals and human eyes showed that there is the only route for the aqueous humor (AH) to flow through its structure [35]. AH gets to the lens only and always through the anterior capsule epithelium and, on metabolism completion, the wasted AH outflows from the lens capsule into the retrobulbar space by means of diffusion through the anterior mini-lens, 0.001 mm in thickness (Fig. 4). If we draw an analogy with the fibrous tunic of the eye (FTE), that has a function of fluctuation, by means of which FTE, actually, squeezes and press out the wasted AH through the biological filters of the eye by diffusion [32, 35], it must be admitted that the lens also has a kind of “breathing”. In particular, in completely near vision when the tension in the lens is increased, the lens is better suited for removal of the wasted intralenticular AH into the retrobulbar space. In other phases of accommodation, intralenticular pressure becomes lower and, in these moments, the lens is easier “to accept” the fresh AH inside through the epithelium for metabolism maintenance. Speaking in simple words, when working with medial and far distances, an emmetropic eye normally “engorge” the aqueous humor inside the lens while, when working with close distances, it removes the wasted AH by diffusion through the posterior mini-lens. This is what we call “the breath of the lens”. Such theoretical ideas lead to important practical conclusions. In case when the eye with acquired myopia is prescribed optical under-correction, the ciliary muscle (CM) is fully or partially slackened when viewing objects at medial and far distances and the amount of the aqueous humor produced by the ciliary processes is significantly decreased. For instance, when looking at distant objects, the amount of the AH produced by the ciliary processes decreases three times as compared with that when looking at close object (CM gets three times less blood) [2]. This can essentially decrease the amount of ingredients which are delivered for healthy metabolism in the lens. There appear important backgrounds for accelerated development of cataract in myopia patients with such irrational optical correction. Vise versa, in mild under-correction for distant vision in hyperopia, residual hyperopia of 0.12-0.25 D, even when working with medial distances, will lead to a close-to-medial tension of the CM when production of the AH in the ciliary processes is within the normal ranges. This is favorable for nutrition of the lens since, additionally, the pressure inside the lens capsule will not be maximal, which eases the absorption of the fresh AH through the anterior capsule epithelium. There are no physiological grounds for accelerated development of cataract. This is why such rational bearable binocular correction should be used to provide far vision both in hyperopes and myopes. It is very important to perform rational optical correction in paired eyes with different refraction precisely in such a way that both eyes have the same values of residual mild over-correction to provide effective binocular work of both eyes and to prevent exophoria. But the main thing in the skill of rational correction is to provide the possibility to completely realize the whole functional working range of the ciliary muscle in the norm, i.e. to provide the recovery (performance) of the whole physiological range of accommodation. And to diagnose the achievement of this key physiological condition, ophthalmologists already have a wonderful unit, an accommodograph. This is how, with the viewpoint of ocular physiology, nightwear ortho-k lenses correct myopia: an early effect of the presence of mild hyperopia is added with a long-lasting effect of activation of the uveascleral outflow which provide healthy metabolism of the collagen structures of the posterior pole of the sclera and, in fact, dwarf axial acquired myopia through excluding an adaptive mechanism of the axial length growth [8, 11-13, 15, 16, 20, 22, 23, 26-28, 31]. Now, let us consider how comfortable near vision is provided. In this case, two tasks should be solved: to provide normal metabolism in the lens as well as in the structures of the intermediate and posterior parts of the eye in order to prevent the development of cataract, myopia or dystrophical changes in the retina in high myopia. For this, a usual mechanism of adaptive axial length adjustment to increased visual load should be guidedly “switched off” [22, 23, 31]. In other words, proper optical correction must bring the tension of the ciliary muscle to the close-to-medial condition which we have long determined as “pre-setting of accommodation" [18,19,22,26]. Physiologically, it can be achieved by mild over-correction for near vision by 0.12-0.25 D both in hyperopes and myopes. However, a very special statement should be made here. A number of internet users worldwide have reached 3.5 billion today, which leads the problem of computer vision syndrome (CVS) and pandemics of shortsightedness to a whole new level because of need to permanently use the displays. Long-lasting display visual load, which can reach in metropolis residents from five to twelve hours a day, will, certainly, lead to a functional fatigue of the ciliary muscle, which has long been classified as a disease in many developed countries and is considered as an insurance event. This refers to computer visual syndrome. There is only one successful method for its prophylaxis so far which is rational optical correction or prophylactic glasses for near vision. And we should specially emphasize that this is even for healthy eyes. Today, we have got adjusted to use additional prophylactic optical correction and/or yellow light filters for the healthy eyes for watchmakers, micro-assembly workers, surgeons, long-distance drivers and so on. Worldwide, workers of many companies and government entities make no bones of wearing the yellow light filters, when working with the displays, that really reduce the visual load. In many entities, it even becomes a code of conduct which decreases the expenses for treatment and payment of insurance fees according to critical diseases/conditions (CDC) as well as increases working efficiency. These preventive measures significantly decrease the chance of CDC appearance. It is clearly necessary to make a new step and to turn to common and compulsory prophylactic correction taking into account the rates of spread of “shortsightedness pandemic”. And this is a worthy task for optometrists worldwide. So, rational optical correction for long-lasting near work should definitely consider a possible fatigue of the CM by the end of the working day. For “display fans”, it can be done through using at once “zero-correction” by 0-0.12 D for near vision. This, certainly, should be chosen individually; however, such approach, according to our clinical data, is effective for myopes [8, 9, 31] and, likely, can be rather effective for hyperopes as well. 2. Optics of the anterior eye Characteristics of the optics of the anterior part of the eyes have been described in details in the previous paper [13]. However, for a deeper understanding of the issue, which is under consideration in the present article, it is necessary “here and now” to explain once more the following. There are two solidly conjugated lenses on entering the eye: the convex-concave tear film with the corneal epithelium and the convex-concave cornea. In the center of the anterior and posterior surfaces of the lens capsule, there are two convergent mini-lenses: the anterior mini-lens and the posterior one which change their optic power in dependence on the shape of the lens, i.e. on the tension of the ciliary muscle. The mini-lenses provide the maximal refraction when viewing near objects. In the center of the posterior surface of the lens capsule, there is a retrolenticular space which is limited by the Wieger's ligament and filled by the metabolism-wasted aqueous humor removed from the lens. This space, likely, additionally provides the optical parallelism of a narrow cone of the RGB-bands to get right on the foveola. We have got adjusted to consider that the light rays entering the eye are refracted only by the cornea. However, that is not so. The incoming light rays first pass through the first “liquid” lens, the tear film with the epithelium, which has a certain thickness. And only then, they pass through the second lens which is the cornea. There is no space between these two lenses and they, in fact, are solidly contacted, making up a kind of biological combined optical system: “tear film + epithelium + cornea”. Such construction of the optics of the anterior eye makes it possible to make a kind of “optic compression”, i.e. “to gather” in the eye a wide picture of the visual space into a narrow “optical tunnel” as in a telescope. This is so because before these rays getting to the lens it is necessary to provide the possibility of their passing through the even narrow pupil. Since the tear film solidly contacts with the cornea (without air space) and the index of refraction (IR) of the cornea is higher by 0.04 than that of the tear film, the light rays, having already been dispersed and compressed in the narrower beam by the tear film, will undergo additional compression inside the cornea when getting into it (Fig. 5) [13].
Let us remind that the cornea is two times thicker in the center than in its periphery, which is a characteristic of a divergent lens. The cornea borders on the aqueous humor of the anterior chamber, the IR of which is less by 0.05. So, the rays coming out of the cornea will be weakly divergent so far (Fig. 5). Since the cornea is a weakly scattering lens, it is also can be seen in Figure 5 that externally imposed color light rays will be slightly scattered in the light tunnel on their passing from the cornea to the aqueous humor while the white light rays will be additionally dispersed in this light tunnel. Such features of boundary optics of the eye make it possible to “drive” an incoming wide light front even through the narrow pupil and, if necessary, to engage effectively the optic part of the lens periphery (a big blue arrow). In other words, there will always be the rays of all spectral color lights. This also refers to the externally imposed color light rays which will be refracted by the optics of the anterior eye but will not undergo dispersion anymore. Conclusions. The cornea itself borders with a less dense optical medium, which is the aqueous humor, and acts as a lens that provides in the eye, at first, an additional compression into “the light tunnel”, and then, on exit, a weak scattering of the light rays having passed through it. That is why the white light rays will undergo dispersion at the posterior surface of the cornea and go to the anterior surface of the lens as slightly scattering and spreading color bands including three basic bands: red, green, and blue (RGB-bands) which can be detected by the cones in the macula. Actually, this is just the feature of the anterior optical part of the eye that makes it possible for the light rays, due to its composition, to pass through the narrow pupil and to separate three basic RGB-bands out of it [12, 13]. As we have underlined in our previous papers, the influence of the thickness and topography of the tear film with the corneal epithelium on the common reflective power of the eye is quite noticeable. This is so because the nightwear ortho-k lenses change, first of all, spatial geometry, thickness, and optical refractive power of the rather viscous epithelium and, hence, the optical power of the tear film, which is not less viscous, by means of the increased thickness of the epithelium in surrounding and the decreased one in the center. By the end of the day, the epithelium and the tear film gradually recover their usual topography, i.e. recover the ability to more effectively compress the light rays, coming into the eye, into “the light tunnel”. Herewith, in the morning, on taking the ortho-k lenses off, the myopic eye obtains mild hyperopic refraction for a while, which makes it possible to successfully retard or to effectively control acquired mild and moderate myopia. It is important to understand that orthokeratology, without changing the geometry of the cornea itself, changes the spatial topography and, hence, the refractive power of the corneal epithelium and the tear fluid. That is why orthokeratology can be considered as not only a reversible but sparing action. 3. Review of ophthalmologists’ opinions on the cornea as a possible main refractive structure of the eye Now, having a detailed consideration of the features of the anterior eye optics, we can assess the fantastic inalterability of a myth among some ophthalmologists, optometrists, and even opticians that the cornea is believed as the main refractive structure of the anterior eye. As we have already cleared it up, anyone who is aware of the physical optics, at least of the secondary school course, finds it obvious that the cornea in its shape is a divergent lens rather than a convergent one. In fact, this is a meniscus that gets thicker from the center to the borders. However, for some reasons, in many websites and references for ophthalmology specialists there is a common misconception of the cornea as “a strong plus lens”. Even the official website of one of the most respectable Russian ophthalmic clinics, unfortunately, gives the incorrect statement, ‘The cornea, optically, is a strong convergent lens’. We have just found out that, when considering the optic system of the anterior eye, we should not speak of the cornea as an isolated lens but the whole “tear film + epithelium + cornea + anterior chamber aqueous humor” system. Indeed, the optical action of the cornea should be considered with due regard to media surrounding it: the epithelium and the tear fluid anteriorly and the anqueous humor posteriorly. To our best knowledge, this issue has once been mentioned in the Russian scientific literature, in particular, the monograph by R.M.Tamarova, Cand. Sc. (Tech) which is titled ‘Optical devices for examination of the eye’ (1982) [36]. R.M.Tamarova writes, ‘The cornea is a cover of almost the same thickness which is slightly thicker in its periphery. This means that an isolated cornea acts as a weak minus (divergent) lens, which is quite unexpected at first sight. According to calculations, the refractive power of the isolated cornea of a mean eye is 5.48 D and its anterior and posterior focal distances are f = f' = -18.25 mm. These calculations refer only to the isolated cornea surrounded by air. In the live eye, the cornea exists in quite opposite conditions. Only the anterior surface of the cornea borders on the air while the posterior one contacts the aqueous humor of the anterior chamber, the index of refraction of which differs little from that of the cornea. Hence, light rays, having come into the eye and passed the cornea that refracts them towards the optical axis, almost do not change their direction when entering the aqueous humor. In such conditions, the cornea acts as a strong plus (convergent) lens and its anterior and posterior focal distances differ: f = -17.055 mm and f' – 22.785 mm. The refractive power of the cornea as a component of the ocular optic system (Dioptric power, Dp) is 43.05 D. The fact that the anterior focal distance is minus and the posterior one is plus points that the lens acts as a plus one’ [36]. In what follows, the author demonstrates the importance of the environment using a spectacular example, as though, of how the refraction of the eye changes when swimming without goggles under the water. It looks like the author has been scared of an unavoidable practical conclusion about a slightly divergent (or less convergent) beam of light when coming out of the cornea and tried to excuse a widely-spread opposite point of view, turning miraculously the cornea into the convergent lens using “holy water”, which is the aqueous humor, and pseudo-scientific figures. Herewith, the author makes another specific mistake ignoring completely the most important role of the tear film with the corneal epithelium. This is so since the cornea solidly borders on them anteriorly without air space. The thin tear film with the epithelium is the first lens of the optic system of the eye. And this plus meniscus powerfully gathers the scattered light rays, coming into the eye, into a beam (“optic tunnel”). This is why the tear film in the area of the cornea should be called “a tear meniscus” with a precise physical (optical) meaning of the term. However, the term of the tear meniscus in the world literature has been accepted, for some reasons, to use as a synonym to a tear prism which is the accumulation of the tear near the lower eyelid. This is a bright example of a failed term which leads to misconception: a proper optic term has incorrectly been transferred into physiology of the tear film and the “tear meniscus” itself has turned from the double concave lens into the prism at the lid margin. Foreign textbooks and references for ophthalmologists and optometrists always point that the cornea is a divergent lens and that, when coming out of it into the anterior chamber of the eye, the gathered beam of light is slightly scattered; the whole “tear film + cornea + aqueous humor” optical system has a plus power in sum. A very popular American textbook by Richard Snell and Michael Lemp, which is called Clinical Anatomy of the Eye, gives correct measurements of the cornea (Table 1) [40].
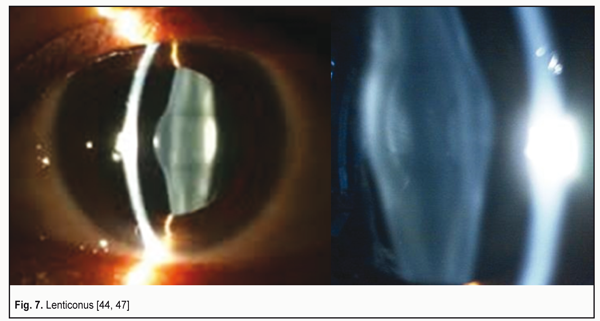
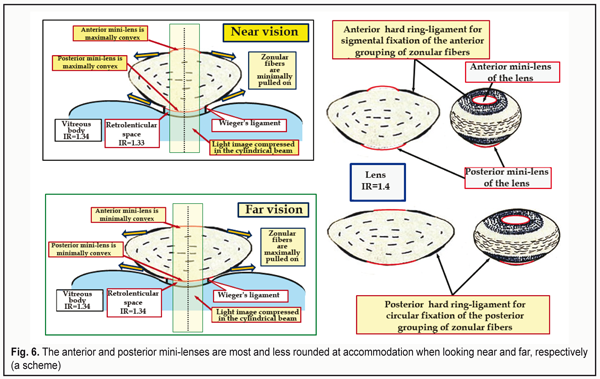
A detailed explanation of these data is given in the paper of M. Talu et al. (2011), ‘Central dioptric power of the cornea (43 D) is a result of the total dioptric power of three optical surfaces (air – tear film: +43.6 D; tear film – cornea: +5.3 D; cornea – aqueous humor: -5.8 D). The cornea is a typical example of a scattering meniscus which is in a contact with transparent media having different indices of refraction’ [45]. Let us underline it once more: this is not a single “powerful convergent lens” but a total refractive power of the three optical surfaces, the latter of which is the divergent one. Some ophthalmologists and optometrists groundlessly state that the difference between the refractive indices of the cornea and the aqueous humor is so negligibly small that these media act, in fact, as “a single lens”. This can be easily refuted by calculating the reflective power which is given in Clinical optics primer for ophthalmic medical personnel: A Guide to Laws, Formulae, Calculations, and Clinical Applications, an American handbook [44]: The refractive index of the cornea, n1 = 1.376; the refractive index of the aqueous humor, n2 = 1.336. The refractive power of a refracting surface is calculated according to the formula: P (D) = 1000 х (n1 – n2) : r [mm], where r – radius of its curvature. Then P (D) = 1000 х (1.336 – 1.376) : 6.8 = - 40 : 6.8 = - 5.8 D. The author of this handbook, Aaron Shukla, an adjunct professor and a leader of the Ophthalmic Technique Program at St. Catherine University (St. Paul, the USA) underlines that the greatest plus power is provided by the air-tear curved surface and the dry cornea without the tear film provides a blurred image. That is why patients with dry eye syndrome suffer from blurry vision, ‘When the surface of the tear film is disrupted, a substantial amount of plus power (+43D) is lost, resulting in blurring due to induced hyperopia. Blinking or instilling nonprescription artificial tears restore the curved surface with its powerful refraction [44]. Three intermediate conclusions: 1. The first “strong convergent lens” with the refractive power of +43.6 D is a compound optic tear layer on the corneal surface that contacts with the air and consists of the tear itself and the transparent corneal epithelium. 2. Since there is no air between the first convergent lens and the anterior surface of the cornea, the incoming light rays, compressed into the beam by the first convergent lens, undergo the additional compression into “the light tunnel”, which has a cylindrical shape with a diameter of 1.8-2.5 mm; the light rays are compressed at the anterior corneal surface by the second compound biological lens (tear film + epithelium + cornea) with the refractive power of +5.3 D. 3. When passing from the posterior corneal surface to the aqueous humor of the anterior chamber, which is the third biological lens of the anterior eye, the white light undergoes dispersion and refraction. The color light rays coming from the surrounding space are also refracted but not dispersed so far. And the cornea itself, as befits according to its geometrical shape, acts as a weak divergent lens with the refractive power of -5.8 D, contacting with a weaker optical medium: the aqueous humor, the refractive power of which is less by 0.04. In the Western world, this is not even a subject of discussion but a paradigmatic fact from textbooks. 4. Optical structures of the lens This is from a school optics course that we know that the lens is a one piece convergent lens without any other refracting surfaces in its capsule. However, this is far from being a full picture. Figure 6 demonstrates the configuration of especially important optical structures of the lens: anterior and posterior mini-lenses at extreme phases of accommodation. Let us note that their refractive power (curvature level) depends on the pressure in the lens that, in turn, depends on the CM tension adequate to the visual load. When viewing close objects, the pressure in the lens and the refractive power of the mini-lenses are maximal while those are minimal when looking at far distances. This is associated with the fact that, at short distances, the lens capsule is minimally stretched by the ciliary zonule and, thus, it more forcefully flattens the lens masses. The presence of the anterior and posterior mini-lenses, 0.05 mm and 0.001 mm in thickness, respectively, is not in doubt. A disease of this lens structures, resulting in lenticonus development, is well known (Fig. 7) [46, 47].
However, what do these mini-lenses with the controlled refractive power exist in the lens for? We have found out that the optic system of the eye is designed in a way that a spatial light stream, entering the eye, is compressed into the cylindrical tube, “the optical tunnel” with a diameter of 1.8-2.5 mm, which is outlined in Figure 6 in a form of the cylindrical tube with a light image compressed into the beam. And this compressed optical image must be delivered right to the macula for its following processing.
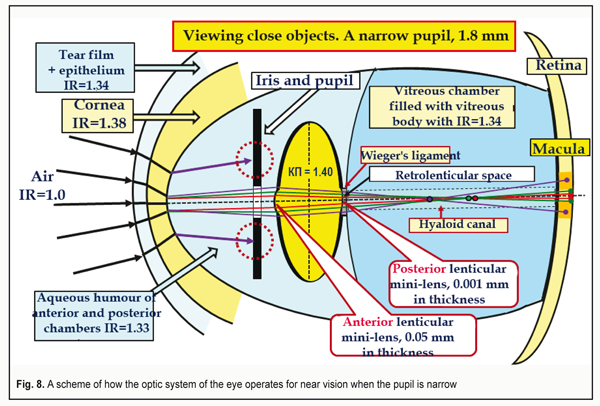
Figure 8 demonstrates this pathway as a scheme of how the light stream, coming from well-illuminated surrounding and being compressed into the cylindrical “light tunnel”, passes through the narrow pupil when looking at close objects.
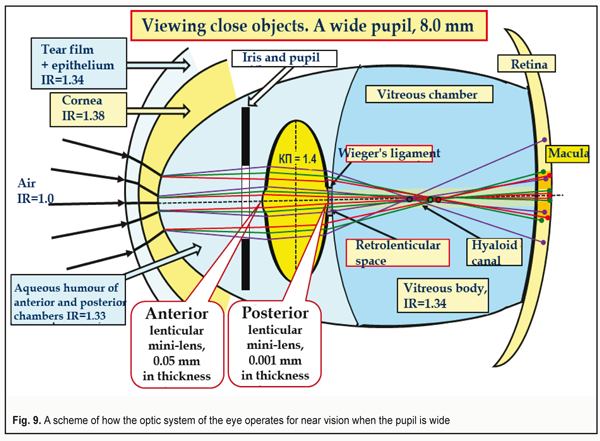
The compressed light stream passes as along the tunnel through the anterior and posterior mini-lenses of the lens and is refracted in them in such a way for the macula to be able to perceive the image as three basic RGB-bands [12. 13]. We should also pay attention to the area of the hyaloid canal in which the lens wasted aqueous humor with metabolism products of the intralenticular structures comes out of the retrolenticular space. Figure 9 shows a scheme how the ocular optic system acts at short distances when the pupil is wide, i.e. under low light conditions. We specially “collect” the foci of coming-through-the-lens lights of each color into one point, which is common for school textbooks. But this simple design is obviously incorrect since the thickness of the lens capsule is different throughout so a braking rate of each RGB-front will be maximal in the center and maximal in the peripheral part of the lens.
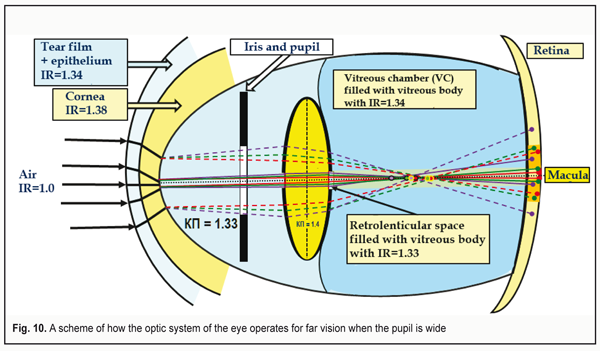
In other words, the fronts of red and green light rays will “run away” both from each other and from the blue front in the central part in a greater degree than in the periphery. There will occur an additional axial but lenticular aberration. And manufacturers of IOL can and must take away this negative part of aberration by optimizing the constructions of contact lenses and IOLs using a graded decrease in their optical dense from the periphery to the center. In fact, this can be IOLs of new generation. Figure 9 demonstrates a maximally wide pupil: 8.0 mm. It is seen that the peripheral light rays cannot get into the macula in this case. The eye is able to detect only rather close objects which are relatively more illuminated and placed centrally to the optic axis. Such “tunnel vision” makes it possible to detect only the closest objects. This condition in the optometry practice is called “night myopia”. Herewith, the lens is set down backwards, compressed and solidly contacts the vitreous body (VB). The volume of the lens masses is constant; the lens capsule is maximally stretched. Inside the lens, the pressure is minimal. Its anterior and posterior mini-lenses have the maximal radius of curvature. When looking at far distance, the tension of the zonular fibers is maximal. I. e. in far vision, the posterior grouping of the fibers stronglier compresses the VB where the pressure is maximal at this accommodation phase [19]. Another bright feature of this case is that the RGB-rays passing through the lens capsule periphery can avoid getting to the macula at all and will not be able to enhance the excitation of its cones. Besides, the different thickness of the lens mass in the center and in periphery results in a different mutual gap between the RGB-wavefronts, which will increase the longitudinal axial aberration. It will be rather difficult to organize the effective focusing of the whole optic system of the eye in such conditions. Figure 10 demonstrates a scheme of the eye's work for distance with a wide pupil. The foci of the wave fronts coming from the lens periphery are marked as yellow circles. To take these additional background aberrations away, it is required to remove this unfavorable background from the lens periphery. That is why the yellow light filters have become so popular: they decrease the intensity of lenticular and general aberration, “cutting off dispersion” of the peripheral lens. At that, deep focus, definitely, will be increased, which makes the long-lasting load work more comfortable.
Conclusion. Viewing far objects creates minimal lenticular aberration as compared to near vision. Understanding this fact is especially important for an optometry practitioner since it is reasonable for better visual performance to prescribe such rational correction that will flatten the lens, if possible. And this is possible only in mild undercorrection. A physiological failure of constant strong undercorrection as a way of acquired myopia braking has been spoken previously in our articles, guides, and monographs [8, 9, 11, 15, 16, 19, 22, 23, 26, 31]. However, what is practically important here is that when choosing correction it is better to mistake towards mild undercorrection than towards strong overcorrection. And this completely refers to planning refractive surgeries for the cornea as well. Practically, this means that rational optical overcorrection when working at various visual distances should be minimal: it should not exceed 0.12-0.25 D for a to-date standard trial lens set and even 0.05-0.07 D if there appear more sensitive methods of detection of changes in visual perception. We should note that highly-sensitive optometric test sets of new generation for visual acuity testing and successful correction selecting can be designed using the principle of the changed level of near/far lens aberration. Certainly, the efficacy of rational optical correction selection should be controlled using an accommodograph enabling to objectively confirm, when such condition is achieved, when the whole individual physiological range of accommodation is involved. We should draw the attention of the optometry practitioners that the comfort visual work also assumes that “the display fans” and patients with impaired vision always have to use, in long-lasting work, the maximally bearable level of the background illumination near the display to reduce the pupil’s diameter, to decrease the level of aberration, and to increase deep focus. These simple recommendations should be obligatory in the practice of any optometrists to prevent visual fatigue and/or acquired myopia. Such “light” recommendations would be also useful for both glaucoma patients and patients with other eye diseases. And here, it is important to use only those video-safe light resources which have radiation spectrum close to the sun one (which, particularly, the retinal cones and rods are set to). But one should be especially careful when using modern energy-saving light-emitting diode resources with a dangerously increased blue level in the spectrum which have captured the world market and which lead to high-level “visual pollution” in metropolitan cities and even in the regional centers [1, 13]. The fact that today on the Earth there have appeared “the light reservations” where it is forbidden to use strong artificial illumination and where it is possible to see the beauty of the sky of stars indicates that there is a need to develop, as soon as possible, the regulations for visual work which are not available worldwide yet. It might be that the optical system of two mini-lenses is the main mechanism of the lens accommodation which makes the mini-lenses more convex when looking at near and more flat when looking at far distances. The mechanism of the “tunnel” accommodation is a changing pressure inside the lens which is maximal when viewing near when the elastic lens capsule is slightly stretched and it can more forcefully compress the lens masses. All that have been said above might be important for a theory and practice of optometry. Taking into account that our clinical outcomes with long-term follow-up periods have confirmed our theoretical ideas, we propose to the optometry practitioners to review some examples of how rational optical correction was used for acquired myopia using the trial lens set with a step of 0.25 D. If there are the trial lenses with a step of 0.12 D, 0.25 D correction should be changed onto this value. Unfortunately, mass production of glasses lenses with gradation of 0.12 D is often troublesome so far. 5. Examples of acquired myopia correction The task of individual optical correction consists in the following physiological action: adjusting the muscular balance, activating the uveascleral outflow pathway when viewing far/close objects and leveling the consequences of possible physiological fatigue of the ciliary muscle in case of long-lasting load work during 6-12 hour a day. Case 1 Myopia, minus 5.0 D We used mild far and near over-correction by 0.25 D in glasses for constant wearing. In case of patient’s professional requirements to provide the possibility of the load work during 6 to 12 hours a day, we start using additional prophylactic glasses with zero or mild undercorrection in the second half of the day, considering the possible physiological fatigue of the ciliary muscle. Rational correction recommended for all patients in this case is constant mild overcorrection for far and near vision: minus 5.25 D. For the patients with constant load work during 6 to 12 hours a day, we use the additional prophylactic glasses with zero or mild undercorrection in the second part of the day, considering the possible physiological fatigue of the ciliary muscle: minus 4.75-5.0 Nota bene! This is how the physiologic action of the nightwear ortho-k lenses is provided. According to the world statistics, ortho-k lenses give the minimal level of acquired myopia progression even in people constantly viewing close objects during and after their work. Case 2 Myopia, minus 5.0 D with exophoria of 4 (up to 5) prism diopters (PD) An additional priority physiological task is to level and brake exophoria development. We use mild over-correction by 0.25 D. Rational correction recommended for this case is minus 5.25 D constantly for near and far vision. Case 3 Myopia, minus 5.0 D with esophoria of 3 (up to 5) PD An additional priority physiological task is to level and brake esophoria development. Rational correction recommended for this case is progressive glasses: minus 5.0 D in a zone for far vision with reading add of 1.5-2.0 D. Conclusions 1. Optical anterior part of the eye is a compound biological lens of three local lenses that includes the refractive structures as follows: the tear film with the corneal epithelium, the cornea itself, and the aqueous humor. 2. The main task of the anterior optics of the eye is to effectively compress the incoming light into “the light tunnel” with a diameter of 1.8-2.5 mm to make it possible to get it even through the narrow pupil. It is a kind of natural telescope. 3. The incoming white light rays get dispersed when coming from the corneal tissues into the aqueous humor of the anterior chamber, the refractive index of which is less than that of the cornea. Of the spectrum, there can be marked three basic bands of red, green, and blue colors which can be detected by the cones of the macula. 4. The cornea itself is a slightly divergent lens. That is why, the light rays, being dispersed at the border on the aqueous humor of the anterior chamber, undergo a slight deviation outwards at the posterior corneal surface. This makes them partially to get even to the lens periphery when the pupil is narrow. The incoming rays of the color light undergo only refraction but not dispersion. 5. There are two convergent mini-lenses, anterior and posterior ones, in the lens capsule placed centrally according to the optic axis; they change their curvature (the refractive power) at different phases of accommodation since the pressure in the lens changes in various tension of the ciliary muscle. 6. Additional lens aberrations are maximal when the pupil is wide and minimal when the pupil is narrow. The light filters make it possible to partially cut off the lens aberration, to increase the deep focus and to make visual load more comfortable. 7. Prophylaxis of myopia, cataract, and other eye diseases consists in maintenance of the metabolic processes in the all structures of the eye through selecting rational optical correction which provides the close-to-medial tension of the ciliary muscle, the sufficient level of the aqueous humor production and uveascleral outflow. 8. The additional measures which can be effective for prevention of computer visual syndrome and adaptive myopia in the healthy eyes are bearable increased illuminating with artificial light sources, close to the solar spectrum, and/or the light filters enabling to increase the comfort visual performance through increasing the focus deepness. References
|