J.ophthalmol.(Ukraine).2017;6:16-19.
|
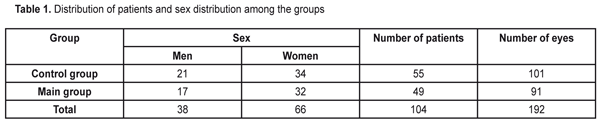
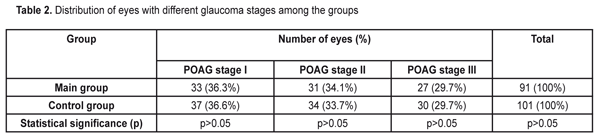
https://doi.org/10.31288/oftalmolzh201761619 Influence of the fetal neuropeptide complex on changes in retinal light sensitivity over time in patients with primary open-angle glaucoma N.V. Panchenko, Dr Sc (Med), E.N. Gonchar, Cand Sc (Med), G.S. Arustamova, MD, A.S. Pereiaslova, MD, D.O. Prikhod’ko, MD, M.V. Friantseva, MD Department of Ophthalmology, Kharkiv National Medical University Kharkiv, Ukraine E-mail: panchenko0802@gmail.com Purpose: To investigate the influence of a fetal neuropeptide complex on changes in retinal light sensitivity over time in patients with primary open-angle glaucoma (POAG). Materials and Methods: The study involved 104 patients (192 eyes; age, 52 to 58 years) with medically controlled POAG and diagnosed with stage I, stage II or stage III glaucoma. In patients of the main group, the fetal neuropeptide complex (Cerebrocurine; Pat. of Ukraine №102,957 issued in 2015) was used as an adjunct to conventional hypotensive therapy. All patients underwent eye examination including visual acuity assessment, biomicroscopy, tonometry, and optical coherence tomography. Changes in retinal light sensitivity in patients with POAG were investigated through changes in mean deviation (MD) using automated static perimetry. In both groups, MD was assessed both before and after treatment. Results: After treatment, the incidence of stabilized MD in patients in the main group was significantly higher than in controls (72.5% vs 45.6%, p < 0.05). The highest and the lowest indices of stabilized MD were seen in patients with glaucoma stage I and glaucoma stage III, respectively (84.8% and 51.8%, respectively). Conclusion: In POAG patients, after the use of fetal neuropeptide complex as an adjunct to conventional treatment, the incidence of stabilized average deviation of retinal light sensitivity was statistically significantly (1.59 times) higher than after the use of conventional treatment only. Keywords: primary open-angle glaucoma, fetal neuropeptide complex Introduction Glaucoma is considered a public health problem and one of the leading causes of irreversible blindness, which contributes to ever increasing incapacitation rate [1-5]. In addition, primary open-angle glaucoma (POAG) is a progressive disease with a steady course, despite a variety of treatment regimens [6, 7]. Efficacy of drugs available for the treatment of glaucoma leaves much to be desired. The incidence of disease progression over at least 3 years after treatment varies from 22.7% [8] to 45.2% [9] and, and over 5 years after treatment, increases to 62% [10]. Nitta K. et al [11] reported that over a mean follow-up of 8.2 years, enlargement of retinal nerve fiber layer defects was detected in 55/93 (59.1%) of eyes treated for normal-tension glaucoma. Wilson et al [12] reported that the percentage of untreated POAG eyes showing progression over 10 years was 53.5% by Advanced Glaucoma Intervention Study criteria and 72.5% by Collaborative Initial Glaucoma Treatment Study criteria. The Early Manifest Glaucoma Trial [13] compared the effect of immediately lowering the intraocular pressure (IOP), vs no treatment or later treatment, on the progression of newly detected open-angle glaucoma. Over a median follow-up period of 6 years, progression was less frequent in the treatment group (45%) than in controls (62%) (P =.007) and occurred significantly later in treated patients. Therefore, therapy for POAG markedly decreases the incidence of glaucoma progression, and the development of innovative methods for treatment of the disease is of primary importance. A fetal neuropeptide complex (Cerebrocurine) derived from fetal bovine brain tissue activates energy and protein production in neurons, improves neuron synaptic function, has nootropic-like and vasoactive effect, and improves cerebral venous and arterial circulation, and seems to represent a promising therapeutic option (Instruction № UA/7516/01/01 on the use of Cerebrocurine dated 14.12.2012). The study purpose was to investigate the influence of a fetal neuropeptide complex on changes in retinal light sensitivity over time in patients with POAG. Materials and Methods The study involved 104 patients (192 eyes; age, 52 to 58 years; mean age, 62.5±3.9 years) with medically controlled POAG and diagnosed with stage I, stage II or stage III glaucoma. The main group involved 49 patients (91 eyes; mean age, 61.2±2.8 years), and the control group involved 55 patients (101 eyes; mean age, 63.9±3.1 years). In patients of the main group, the fetal neuropeptide complex (Cerebrocurine; Pat. of Ukraine №102,957 issued in 2015) was used as an adjunct to hypotensive therapy. There was no significant difference in sex distribution (Table 1) or distribution of POAG stages (Table 2) among the groups.
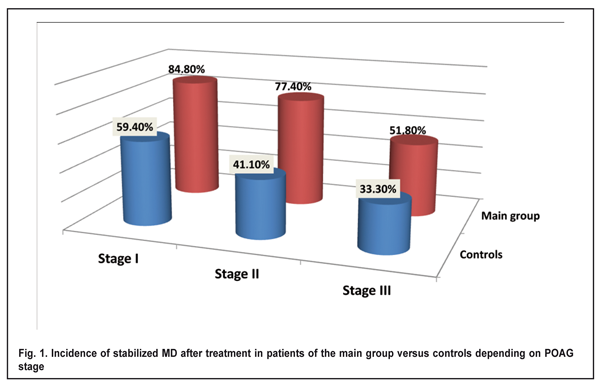
POAG stage was determined by a reduction in retinal light sensitivity with mean deviation (MD), and eyes were classified (as per Volkov’s classification of POAG [7, 14]) as those with stage I glaucoma (early glaucoma; MD ? -6 dB), stage II glaucoma (moderate glaucoma; MD, -6.01 to -12 dB), and stage III glaucoma (advanced or severe glaucoma; MD, -12.01 to -20.0 dB), based on the number of points affected, i.e., with reduced retinal light sensitivity. All patients underwent eye examination including visual acuity assessment, biomicroscopy, tonometry, and optical coherence tomography. In addition, changes in retinal light sensitivity in patients with POAG were investigated through changes in MD over time using automated static perimetry (OCULUS Twinfield Version 3.15r07 program 30-2 full-threshold test; OCULUS Optikger?te GmbH, Wetzlar, Germany) [4]. In both groups, MD was assessed both before and after treatment. Treatment outcome was defined as stabilization if the post-treatment MD was not lower than baseline. Treatment outcome was defined as worsening if the post-treatment MD was lower than baseline. The incidence of stabilized average deviation of retinal light sensitivity was defined as a percentage of eyes in which the post-treatment MD was not lower than baseline. Results Hypotensive agents with neuroprotective effects were used in 80.2% of eyes in the main group versus 81.2% of eyes in the control group (p > 0.05). Incidences of stabilized MD in patients with glaucoma stages I, II and III in the main group (after the fetal neuropeptide complex as an adjunct to conventional treatment) and in the control group (after conventional treatment only) are presented in Figure 1.
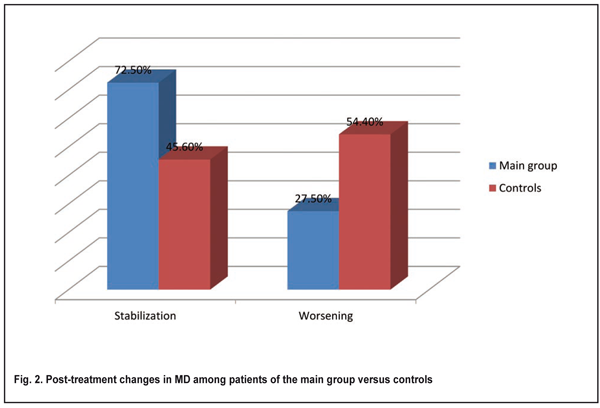
In patients with glaucoma stage I in the main group, the incidence of stabilized MD was significantly higher than in controls (84.8% vs 59.4%, p < 0.05). Therefore, in patients with glaucoma stage I, after the use of fetal neuropeptide complex as an adjunct to conventional treatment, stabilized MD was obtained 1.4 times more often than after the use of conventional treatment only. In patients with glaucoma stage II in the main group, the incidence of stabilized MD was significantly higher than in controls (77.4% vs 41.1%, p < 0.05) (Fig. 1). Therefore, in patients with glaucoma stage II, after the use of fetal neuropeptide complex as an adjunct to conventional treatment, stabilized MD was obtained two times more often than after the use of conventional treatment only. In patients with glaucoma stage III in the main group, the incidence of stabilized MD was higher than in controls, although the difference was not significant (51.8% vs 33.3%, p > 0.05) (Fig. 1). Figure 2 demonstrates post-treatment changes in MD in the main and control groups irrespective of POAG stages. In patients in the main group, the incidence of stabilized MD was significantly higher than in controls (72.5% vs 45.6%, p < 0.05).
Therefore, in POAG patients of the study, after the use of fetal neuropeptide complex as an adjunct to conventional treatment, stabilized MD was obtained 1.5 times more often than after the use of conventional treatment only. Conclusion We found that, in POAG patients, after the use of fetal neuropeptide complex as an adjunct to conventional treatment, the incidence of stabilized average deviation of retinal light sensitivity was statistically significantly (1.59 times) higher than after the use of conventional treatment only. The highest and the lowest indices of stabilized MD were seen in patients with glaucoma stage I and glaucoma stage III, respectively (84.8% and 51.8%, respectively). Therefore, after the use of fetal neuropeptide complex as an adjunct to conventional treatment for POAG, stabilization of visual function was observed significantly more often than after the use of conventional treatment only.
This study (1) gives evidence that the fetal neuropeptide complex is a valuable adjunct broadening the neuroptotective therapeutic armamentarium for POAG and (2) may provide the basis for investigation of the influence of the fetal neuropeptide complex on the indices of retinal and optic nerve structure in patients with POAG. References
|