J.ophthalmol.(Ukraine).2016;6:37-49.
|
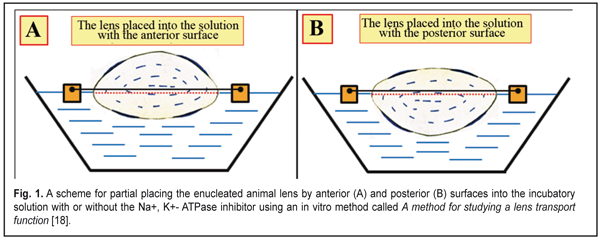
https://doi.org/10.31288/oftalmolzh201763749 Physiological properties of fluid circulation in the crystalline lens in animals with regard to a phase of accommodation Stepanova L.V. 1, Cand Sc (Biol); Sychev G.M. 2, Cand Sc (Med); Kratasiuk V.A. 1, Prof., Cand Sc (Biol); Svetlova O.V. 3, Prof., Cand Sc (Med); 1 Siberian Federal University; Krasnoyarsk (Russia) 2 Katanov Khakas State University, Abakan (Russia) 3 Mechnikov North-West State Medical University; Saint Petersburg (Russia) E-mail: slyudmila@mail.ru. The work was supported by Russian Fund of Fundamental Studies (Project No 16-06-00439). Background. Traditional ideas about the fluid circulation in the lens assume its movement through the lens capsule inside-out and its surface distribution in the lens bulk. It is alleged that "fresh" intraocular fluid directionally diffuses from the posterior chambera into the lens toward its center through both its the anterior capsul epithelium and the posterior lens capsule wall. Then intraocular fluid moves along the lamillar structures of the lens towards the equator, where, likely, the Na+, K+-pump activity is maximal. Removal of "waste" of fluid from the lens bag goes in all directions: either through the anterior and posterior capsule, or through the equatorial part. However, these views do not take into account the physiological characteristics of the transport properties of the anterior capsule epithelium that is capable, due to an existing therein ion exchange system, to provide only the one-way fluid flow: from outside to inside. The possible changes in the level of pressure within the lens in different accommodation phases, which may affect the intensity or direction of water exchange, are also not taken into account. The aim of the research. To identify the main mechanisms of the water-exchange process in the lenses of animals, taking into account a phase of accommodation. Materials and Methods. Lenses of rabbits and cattle were studied. The fluid transport processes in the lenses were examined in vitro by changing their weight when placed in incubatory solutions with or without an inhibitor of Na+, K+ -ATPase. In the first part of the in vitro studies, the lens partially (some lenses with the anterior surface, others with posterior surface) were immersed in solutions representing the lens surrounding medium. In the second part of the in vitro study, the lenses were completely immersed in a solution similar in ionic composition to aqueous humor, with different osmotic pressures. The direction of movement of intraocular fluid was studied in vivo according to dye distribution using biomicroscopy and a stopped diffusion method. Results. The epithelium of the anterior lens capsule of rabbits and cattle supports the water transport from the posterior chamber of the eye into the lens through the work of Na+, K+ -ATPase. This active ion transport system facilitates the directed movement of the "fresh" fluid, rich in metabolites, through the anterior capsular epithelium from the anterior surface of the lens capsule to the posterior one. For the first time we found that at the completely "near" accommodation phase the maximal pressure inside the lens capsule is 6 mm Hg. This state is a dynamic balance for the lens, i.e. the value of the osmotic pressure of 6 mm Hg balances in the lens the level of the mechanical pressure of the capsule. The lens weight right after removing was close to its original weight with the osmotic pressure of 6 mm Hg. When looking completely "far", the lens bag is stretched with a ciliary zonule and minimally compresses the masses inside the lens (minimal intracranial pressure). This contributes to the intensive inflow into the lens of "fresh" fluid. The anterior capsular epithelium of the lens was found to support the water transport from the posterior chamber of the eye into the lens by the active ion transport system Na+, K+ -ATPase. It is important to note that the fluid circulation inside the lens occurs along the osmotic gradient and is always unidirectional from its anterior epithelium to the posterior wall of the capsule. Diffusive fluid circulation inside the lens does not occur through its nucleus, but along the fibers inside the lens, followed by diffusion of the "waste" fluid outward through the posterior surface of the lens into the vitreous chamber. This mechanism of micro changes in the volume and/or fluid replacement in the lens can be thought of as a mechanism of "fluctuations" of the lens volume. Conclusion. The theory of "lens volume fluctuations" at the "near-far" accommodation phases is presented; the theory is confirmed in animal experiments in vivo and in vitro. Understanding this physiological process makes it possible to selectively choose the type of rational correction for more effective inhibition and prevention of the cataract or presbyopic process. Keywords: animal lens, fluid circulation, osmosis, diffusion, pressure in the lens, accommodation, prevention, rational correction, cataract. Background Metabolism in the crystalline lens supports water-exchange process renewing the intraocular fluid through osmotic and diffuse mechanisms [15, 17, 20, 29, 30]. A quite popular existing theory of fluid circulation supposes that “fresh” fluid gets into the lens through anterior and posterior lens surfaces of the lens and then “wasted” fluid gets out from the lens at bthe equator [22, 27-29]. However, glutathione concentration (gamma-Glutamylcysteinylglycine) synthesized in cortical layers of the lens gradually decreases on advancing to the nucleus of crystalline lens [7, 8]. This disproves the idea of fluid movement first to the lens center and, afterwards, from the center to the periphery. Recent studies on fluid movement in isolated lenses have reported that the lens is a pool, in which fluid diffusion occurs in all directions [2, 25]. The data presented have been obtained regardless transport properties of anterior lens epithelium. Anterior surface of the lens capsule is lined from the inside with simple epithelium [5, 15, 17] that contains a system of active ionic transport: Na+, K+- ATPase [4, 26]. This ion-exchange “pump” creates a gradient of osmotic pressure outside and inside the lens by means of various concentrations of К+ and Na+ ions, which contributes to the osmotic movement of the “fresh” fluid inside the lens [21, 23, 24, 26, 27]. Normally, the fluid, “pumped” inside the lens, can unidirectionally duffuse later only from the anterior lens surface to the posterior one. Morphological studies have determined that the lens has a fibrous concentrically-bulked structure which has up to 40 layers at the young age similar to tree’s year rings [5]. The layers have a membrane “petal” ellipsoid design and are separated by intraocular space (0.01 ?m in width) filled in with intraocular fluid [5, 15]. It is possible that diffuse fluid movement in the lens can occur in these intraocular spaces between and along the parallel layers of ellipsoid cortical surfaces. The fluid diffusion in the lens can be directed either outwards only (in norm) or towards both sides by turns and differently toward each anatomical part of the lens capsule. A study on dye transfer between cells of the lens has determined that the dye is distributed along the fibers with the dye concentration lowering from anterior capsule subepithelial fibers to central fibers [30]. The posterior lens capsule is thinnest and adjacent to the retrobulbar space which is formed by the Wieger's ligament and the fossa of the anterior hyaloid membrane of the vitreous [5]. The posterior surface of the lens capsule in the central part, including the area of the posterior mini-lens, is tightly bound with the vitreous chamber filled with the vitreous body [3,5,6,12]. High molecular weight proteins and organic compounds, which are vitreous constituents, can induce in the vitreous high oncotic pressure that can provide a physiological capability to pump inside the vitreous chamber the fluid from outside [3]. That is why the “waste” intraocular fluid from the posterior lens capsule can outflow into the Cloquet canal of the vitreous and into the vitreous body by means of oncotic pressure induced in the vitreous chamber. Studies on fluid circulation in the vitreous have shown that mutual diffusion of the intraocular fluid from the posterior chamber to the vitreous through the vitreous membrane occurs rather freely and in both directions [3, 5]. It is highly likely that the “waste” intraocular fluid outflows from the lens mainly by means of diffusion through the especially thin posterior mini-lens of the lens capsule, which seems to be a more likely physiological pathway. Besides, water exchange processes must be interrelated with physiological processes of the lens accommodation. I.N. Koshits et al. [11] have developed a hypothesis that there is a previously unreported mechanism of the lens accommodation in the eye which is related to quick changes in the reflective power of the anterior and posterior mini-lenses located on the optic axis of the lens and the eye itself. The size of “the standing out” (changeable refractive power) of the mini-lenses at different accommodation phases (when looking near “the standing out” is maximal; when looking at the distance, it is minimal) makes it possible for light beams, gathered into “the optic tunnel”, to precisely focus in the macula while their passing through the corneal center parallel to the optic axis of the eye. The process of “the standing out” can be related to changes in pressure inside the lens and can influence the intensity of osmotic and diffuse transport in the lens despite “a lenticular mechanism” of accommodation which is quicker then biological water exchange mechanisms permanently occurring in the lens. Thus, the existing ideas of fluid circulation in the lens cannot explain the physiological features of interaction of lens accommodation mechanisms and mechanisms of lens bulk transparence maintenance; they also cannot reveal pathways and conditions for metabolic products removal from the lens capsule and give a deeper understanding of physiological features of cataract or presbyopya development By the lows of nature, the lens bulk is incompressible and cannot be dilated at the constant temperature, i.e. its current volume is constant. If the lens capsule did not press out the lens bulk from outside, then the pressure inside the lens would be equal to the pressure in the posterior chamber, i.e. practically to the intraocular pressure. It is important to understand that the “mechanical” part of the intralenticular pressure is formed only by means of lens capsule rigidity that provides the pressing out of the incompressible lens bulk at all accommodation phases. Herewith, the inflow of the “fresh” intraocular fluid through the lens epithelium by means of osmosis must, likely, be balanced in norm by the outflow of the “waste” intraocular fluid by means of diffusion, the intensity of which is also clearly related to intraocular pressure rates. In case of the increased inflow of the “fresh” intraocular fluid into the lens capsule or the low outflow of the “waste” intraocular fluid, the lens volume increases, the lens capsule stretches, the lens rigidity grows up, and, in response, the pressure inside the lens increases. However, a functional expansibility of the lens capsule is achieved by morphological and physiological characteristics of the lens tissues and architectonics and is limited. Thus, when the intraocular fluid volume is increased inside the lens bag, the lens capsule can be stretched only up to the fixed measure, and the further inflow of the “fresh” intraocular fluid is difficult due to thr increased pressure inside the lens. In this case, the increased pressure inside the lens automatically balances the osmotic pressure of the lens, and the diffuse outflow of the “waste” intraocular fluid out of the lens capsule is balanced by the osmotic inflow of the “fresh” intraocular fluid. Herewith, the lens can pump by osmosis so much “fresh” intraocular fluid as a Na+, K+- ATPase concentration inside the lens can allow. This, likely, can occur in norm at any lens capsule stretching degree and at a corresponding pressure level inside the lens. It should also be noted that the diffuse outflow (“pressing out” of “waste” intraocular fluid) through the lens capsule, more likely, can occur not only by means of the action of intralenticular pressure but also by means of oncotic mechanisms in the vitreous, which are studied incompletely so far and which are able “to select” the intraocular fluid out of the lens bag by osmosis. The vitreous can have an osmotic ability to “plump out” of the lens the “waste” intraocular fluid, maintaining lens metabolic processes in norm. These hypotheses require experimental approval. With aging, rigidity of the lens capsule increases, its elastic properties decrease and the lens functional ability to volume fluctuation reduces, i.e. maintainability of metabolic process in the lens gradually decreases. Under certain conditions, there appears a background for cataract development even before presbyopic age (45 to 60 years). Otherwise, the amount of cortex layers in the lens constantly grows throughout life and the lens bulk is twice increased at the end of presbyopic age [5, 6] and the lens capsule is overstretched. And the lens capsule rigidity and responsive intralenticular pressure additionally increase and the level of the diffuse outflow of the intraocular fluid grows up. This also creates significant backgrounds for cataract development since the new “fresh” intraocular fluid can partially be flown out together with the “waste” intraocular fluid. It is known that optical properties of the transparent lens are defined by the constant volume of the lens bulk which consists of fibers, incompressible and unstretching at constant temperature [9, 11, 14], with narrow intercellular spaces filled in with the intraocular fluid [8]. In the absence of accommodation (when looking at far objects), the lens capsule is maximally stretched and the intralenticular pressure is minimal, which eases the pumping of the “fresh” intraocular fluid from the posterior chamber by osmosis. Herewith, the diffuse flow of the “waste” intraocular fluid out of the lens capsule through its posterior wall is difficult [9, 14]. At accommodation when looking at near objects, the lens capsule maximally “presses out” the incompressible lens bulk, which results in the increased intralenticular pressure. The rigidity of the lens capsule creates the additional intralenticular pressure that is higher than the intraocular pressure at all accommodation phases [9, 11, 13, 15]. Herewith, the inflow of the “fresh” intraocular fluid through the anterior capsule epithelium must be balanced by the outflow of the “waste” intraocular fluid through the posterior capsule. Thus, the early ideas of the physiological features of fluid circulation in the lens require specifying the pathways and conditions for metabolic product removal from the lens capsule at different accommodation phases. This can give a deeper understanding of physiological features of maintaining the lens transparence and conditions, under which cataract or presbyopya develop. The purpose of the study was to reveal main mechanisms of fluid circulation in the animal lens with regard to an accommodation phase. Material and Methods Experimental study was carried out on 90 rabbit eyes and 30 cattle eyes. The rabbits (Soviet Chinchilla; weight, 1.5-.03 kg; age, 3-7 months; both genders) were maintained under standard conditions in vivarium of ZAO Krasfarma, Krasnoiarsk. The eyes of cattle (bulls) were collected after commercial slaughter and had no pathological signs. Animal experiments followed general principles of Resolution on the Use of Animals in Research accepted by the Association for Research in Vision and Ophthalmology (ARVO). The lens was enucleated from the eye 40 min after the biological death of the animal. Fluid circulation in the lens was studied under various experimental physiological conditions: in vitro study assumed an accommodation phase of looking at near objects, which could facilitate fluid circulation processes of a weaker intensity; in vivo study corresponded to a condition of looking at distance, which could facilitate more intensive fluid circulation processes. As incubatory solutions, we used a balanced solution, similar to the intraocular fluid in ion content, and “a biological solution”, which was an enucleated vitreous body. The vitreous chamber was removed from the eye together with the vitreous body. The balanced solution, similar to the aqueous humor in ion content (pH=7.4), contained NaCl, 104.32 мМ; NaHCO3, 27.26 мМ; KHCO3, 4.08 мМ; MgCl2, 0.79 мМ; calcium gluconate,1.77 мМ; glucose, 5.55 мМ; K2SO4, 0.59 мМ; cevitamic acid, 0.56 мМ; NaH3PO4?2H2O, 0.28 мМ; Na2HPO4, 0.56 мМ. The transport activity in the lens was slowed down by adding to the incubatory solutions 10-8 M strophanthine K, an inhibitor of Na+, K+- ATPase, penetrating to the lens capsule together with the incubatory solution fluid. Osmotic pressure in incubatory solution was leveled up (from 22 to 60 mmHg) by adding a high-molecular substance, polyvinyl pyrrolidone (PVP) (BASF, Germany) with a molecular weight of 150 Da. Osmotic pressure in the incubatory solution was measured using an OMKA 1Ts-01 osmometer (relative measurement error, ± 1%). The lenses were weighted using a VLR-1 precision balance (relative measurement error, ± 10-3 g). In the first part of the in vitro study, the enucleated rabbit lenses were partially, not in full, by anterior (n=30) or posterior (n=30) surfaces, placed into the incubatory solutions with or without the Na+, K+- ATPase inhibitor (Fig. 1).
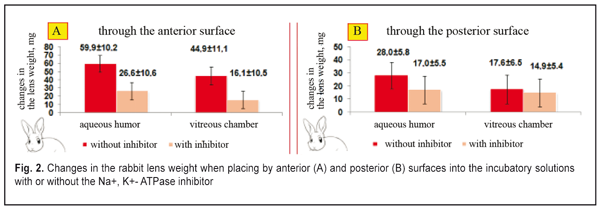
In the second part of the in vitro study, the enucleated lenses (rabbit, n=30; cattle, n=30) were fully placed into the balanced solution with various osmotic pressure levels (from 22 to 60 mmHg) and with or without the Na+, K+- ATPase inhibitor. The studies were performed in a thermostat (36°C) for 60 min. The lens were weighted to determine changes in the lens weight. Fluid circulation in the rabbit animal (n=6) was studied in vivo according to dye distribution using biomicroscopy and a stopped diffusion method. A 10% fluorescein solution was used as a dye. We added a 30 % PVP solution to increase the dye viscosity in order to decrease rates of fluorescein spreading in the lens bulk from the needle injection channel. This made it possible to observe a slow spread and flow of fluorescein along current lines inside the lens. In the in vivo studies, the rabbit eyes were anesthetized by an intramuscular novocaine injection and a dicaine eye instillation. Then, an eyelid speculum was used to keep animal eyes open. 0.001 ml of the viscous dye was administered at 2 mm from the limbus through the equator to the lens center. Online lens distributing and flowing in the lens was observed using an Opton slit lamp (Germany) under mydriasis (the mydriatic imitation of the distant accommodation) at 450 nm. Within an hour, every 5-30 min upon the dye administration, the animals were taken out from the experiment; the eyes were immediately enucleated and liquid nitrogen frozen (at temperature of -186°C). The frozen eyes were cut through the injection site using a microtome Slide 2002 Compact (Germany) and registered using a Olympus Camedia C-50 (China). Statistica 10 (StatSoft Inc., the USA) was used to statistically process the data. Shapiro-Wilk W-test for normality was performed for distribution of the studied values. The data were processed with calculating the mean value (M) and standard deviation (s). Student-t criterion was used to assess the difference significance. The significance was accepted at a level of p<0.05. Results and Discussion Let us consider the organization of animal lens fluid circulation processes at a phase of accommodation when looking at near objects, which we experimentally determined in the first part of in vitro study. Changes in rabbit lens weight in incubatory media similar to the intraocular medium are shown in Figure 2. Cases A and B correspond to near sight (the lens is completely rounded) when the incompressible lens bulk is maximally pressed out and “pressing out pressure” inside the lens is about maximal. The increased intralenticular pressure is likely to slow down the rates of the fluid plumping into the lens from the surrounding water medium by means of ion-exchange transport system.
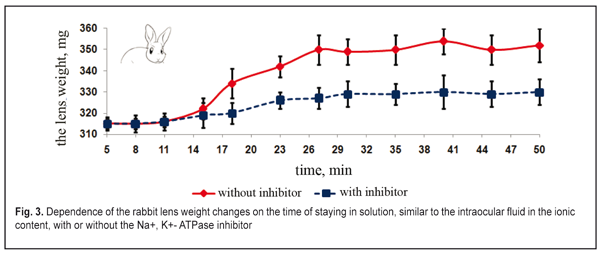
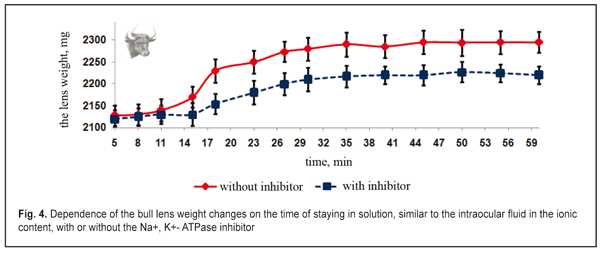
Figure 2 demonstrates that the lens weight is significantly increased after their partial placing by the anterior surface (with the epithelium) into either the balanced inhibitor-free solution, similar to the posterior chamber humor in ionic content, or the vitreous humor (the enucleated vitreous body without the inhibitor). The lenses plumped the intraocular fluid inward from the balanced solution by osmosis. This resulted in an increase of the lens weight, at average, by 16-22% for the inhibitor-free solution and 11-17 % for the solution with the inhibitor (p<0.01). In osmotic “plumping” the intraocular fluid from the vitreous humor, the lens weight was increased, at average, by 16-22% for the inhibitor-free solution and 11-17 % for the solution with the inhibitor (p<0.01). And the lens capsule was extra-stretched and the pressure inside the lens was increased. The osmotic pressure inside the lens being equal to the increased intralenticular pressure, which was caused by an increase in lens capsule rigidity, the transport of the intraocular fluid was stopped since the lens capsule had been stretched due to the increased fluid volume. The animal lenses placed by the posterior surfaces into the same incubatory solutions with or without the Na+, K+- ATPase inhibitor had a significantly less difference in lens weight changes (p > 0.1). The lenses “plumped” less intraocular fluid from the balanced solution by means of diffusion and, partially, osmosis. As result, the lens weight was increased, at average, by 5-12% for the inhibitor-free solution and by 1-8% for the solution with the inhibitor (p<0.05). When “pumping” the intraocular fluid from the vitreous humor by diffusion and, partially, osmosis, the lens weight increased, at average, by 3-7% for the inhibitor-free solution and by 3.1-6.6% for the solution with the inhibitor (p<0.05). The inflow of small amount of the intraocular fluid through the posterior capsule inside the lens was by means of diffusion without the active transport system involved. Thus, the Na+, K+- ATPase inhibitor did not have a significant effect on the water inflow through the posterior lens surface. This enables to conclude that this is diffusion that is mainly involved in the inward or outward fluid transport through the posterior surface of the lens capsule. The inhibiting response to Na+, K+- ATPase resulted in a significant decrease in the amount of inflowing intraocular fluid in the lens, which was observed when the lenses were placed into the incubatory solutions by the anterior and posterior surfaces. This points out the fact that the osmotic transport, conditioned by the active Na+, K+- ATPase transport system, takes the leading part in fluid transporting into the lens. Unlike the posterior one, the anterior surface of the lens capsule is a monolayer of epithelial cells. So, the epithelium of the anterior lens surface participates in the intraocular fluid transport into the lens and determines its movement. The results given coincide with previous data, obtained by Maltsev E.V. and Pavlyuchenko K.P., on the lens epithelium participating in the active transport of the “fresh” intraocular fluid into the lens [15]. Thus, we experimentally proved the transport of the fluid through the anterior capsule epithelium into the lens with Na+, K+- ATPase involved as well as the absence of the active transport of the intraocular fluid into the lens through the posterior lens capsule. In the first part of the in vitro experiment, the increase of the lens weight could be not only caused by osmotic transport with participation of Na+, K+- ATPase, located in the anterior capsule epithelium but be also influenced by the difference in osmotic pressure between the native lens and the incubatory solutions. In the second part of the in vitro experiment, the osmotic pressure inside the lens was measured according to changes in the lens weight when the lens was completely placed in the solutions, similar to the intraocular fluid in the ionic content, but with various osmotic pressures. First, we studied the changes in weight of rabbit and cattle lens placed in the incubatory solution, at first, without the Na+, K+- ATPase inhibitor, then with the inhibitor. The dependence of changes in rabbit and cattle lens weight on the incubation time in the incubatory solution without osmotic pressure was identical (Fig. 3 and 4).
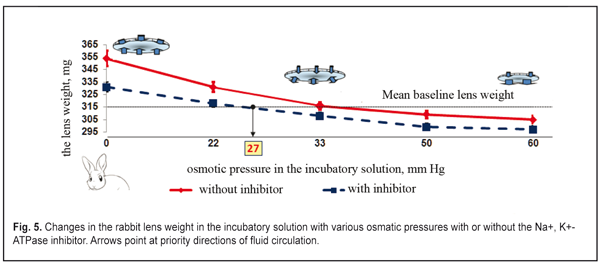
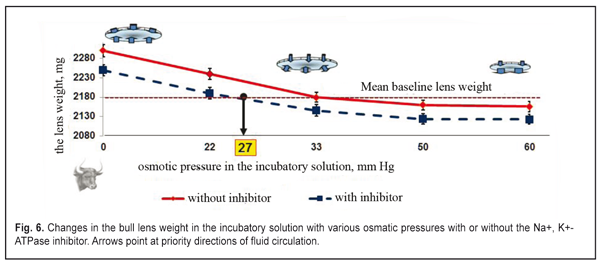
The lens weight in the studied animals changed little, if any, within the first 8-11 min. A significant increase in the lens weight was observed between 15 and 27 min. At half an hour from the experiment start, the lens weight increase was maximal. Afterwards, no significant lens weight increase was observed. The osmotic process was significantly slower as compared to time functional accommodation intervals (split second). In the second part of the study, the lens weight in the incubatory medium with or without the Na+, K+- ATPase inhibitor differed significantly (p< 0.001). A less increase in the lens weight in the presence of the inhibitor was caused by the decreased activity of the osmotic process. No changes in the lens weight at saturation after 30 min can, likely, be explained by the balance between the intralenticular pressure and the oncotic pressure which is created by soluble lens proteins at maximal possible water saturation of the lens. Similar regularities were obtained in the incubatory solutions with various osmotic pressure levels and with or without the Na+, K+- ATPase inhibitor. Figures 5 and 6 demonstrate the influence of the osmotic pressure in the incubatory solution on the changes in weight of lenses completely placed in it. The horizontal lines in Figures 5 and 6 correspond to “the average weight” of the rabbit and cattle lenses at baseline (before the experiment) and are accepted as a zero reading line.
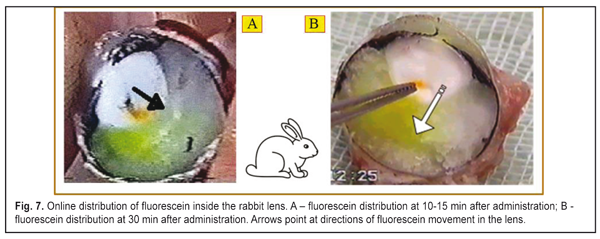
Our data revealed the same dependence of the changes in the rabbit and animal lens weight on the osmotic pressure in the incubatory solution both with and without the Na+, K+- ATPase inhibitor. Figures 5 and 6 demonstrate that when the osmatic pressure in the incubatory solution is increased from 0 to 33 mmHg, the lens weight is decreased due to fluid loss, which is caused by two mechanisms: 1) pressing out the fluid outside by the lens capsule; 2) diffusion of the fluid between the incubatory solution and the content of the lens caused by the different oncotic pressure. A large range of osmotic pressure values from 0 to 33 mmHG was caused by the increased fluid loss in the lens. Almost no changes in the lens weight were noted when the osmotic pressure was 33 mmHg and more both for rabbits and for cattle. When the osmotic pressure in the incubatory solution was ? 33-60 mm HG, the lens weight decreased insignificantly as compared to the mean value. Water loss in the lens, placed into the incubatory solutions with high osmotic pressure levels, was almost the same. So, a decline of the experimental curve, lined from “the bend point” on the “mean weight” line, is significantly decreased. It can be considered that the lens weight even at the high oncotic pressure levels in the incubatory inhibitor-free solution was constant (the right part of the graph, a full line). So, in fact, we observed the constancy of the lens volume because the intraocular fluid was “bound” in the lens due to the oncotic pressuer inside the lens. It is, likely, not only the effect of the intralenticular pressure on the intraocular fluid outflow was decreased but the effect of the high level oncotic pressure in the incubatory inhibitor-free solution. When the lenses were placed into the incubatory solution with the Na+, K+- ATPase inhibitor, the lens weight also increased at the low osmotic pressure levels ? 27 mmHg and decreased at the high osmotic pressure levels ? 27 – 60 mm HG; however, these values were less significant as compared to those obtained in the incubatory Na+, K+- ATPase inhibitor-free solution (р<0.01). The significant difference in lens weight changes in the incubatory solutions with and without the Na+, K+- ATPase inhibitor shows that the active transport system has a determining part in fluid circulation of the native lens even at the near vision accommodation phase. The increased lens weight in the solutions with the osmotic pressure of 0 – 27 mmHg without the Na+, K+- ATPase inhibitor, as compared to the mean lens weight, can be explained by intensive inflow of the intraocular fluid into the lens through the anterior capsule epithelium by osmosis. In addition to this transport, the intraocular fluid could flow into the lens also through the posterior capsule upon gradient of the oncotic pressure created by soluble lens proteins. The lens weight increase occurred despite the response pressure, existing and growing inside the lens. The response pressure is caused by the increased mechanical pressing of the incompressible lens bulk by the lens capsule even if there is no external intraocular pressure. The increase in the mean lens weight in the incubatory inhibitor-free solution with the osmotic pressure of ? 33 – 60 mm Hg gives the evidence of a comparatively small water volume flowing from the lens into the incubatory solution. In this case, physiological features of the Na+, K+- ATPase transport system, likely, were not fully sufficient to fill up the loss of the intraocular fluid and to keep it inside the lens capsule. The addition of the Na+, K+- ATPase inhibitor switched off the activity of the transport system (Na+, K+- ATPase) and the lens notably lost its unbound free fluid because of the drop of the osmotic pressure in the lens and in the incubatory solution (the right part of the graph, dashed line). The constancy of the decreased values of the mean lens weight in the incubatory inhibitor-free solutions with the osmotic pressure of 50 and 60 mm Hg gives us the evidence of a physiological ability to keep a necessary and, perhaps, constant amount of fluid by means of proteins of the native lens. No changes in the lens weigh in the incubatory inhibitor-free solution with a relatively large ocmotic pressure, 33 mmHg, show that in such conditions the fluid transport inward and outward the native lens is balanced. Thus, we experimentally proved that the intralenticular pressure could take an important, but not determining, part in fluid circulation and metabolism in the lens. However, for cataract prevention, rational optical correction must, if possible, exclude a condition of long lasting stressed near vision, changing it to a more comfortable condition when the ciliary muscle has the medium tension and, thus, the pressure inside the lens is not maximal. When the Na+, K+- ATPase transport system was switched off, the oncotic pressure, created by lens protein, was 27 mm Hg (Fig. 5 and 6). And the value of the in-lens ocmotic pressure, created by lens proteins (oncotic component) and the Na+, K+- ATPase transport system activity, is 33 mm Hg. The difference between the values of the osmotic and oncotic pressure is 6 mm Hg and determines the efficacy of the Na+, K+- ATPase activity. Data obtained make it possible to conclude that the active transport system (Na+, K+- ATPase) is able to increase the volume of the intraocular fluid in the native lens capsule plumping in the fluid through the lens epithelium after the lens has been enucleated. Since the lens capsule is stretched by the extra fluid, its rigidity is increased; this results in the response growth of the intralenticular pressure, which facilitates the directed diffuse fluid movement outward with the gradually increasing oncotic pressure in the lens-surrounding solution. In vivo study, when the dye was injected into the rabbit lens, revealed a directed movement of the dye from the anterior capsule lens surface to the posterior one (Fig. 7 A, B). Figure 7A shows that at 10-15 min after the administration, the dye was always distributed from the injection site towards the posterior surface. Hereafter, the dye went out of the posterior lens surface and entered the vitreous body (Fig. 7B). 40 min after the administration, the dye was not visually registered since it, likely, had been completely removed from the lens within this time.
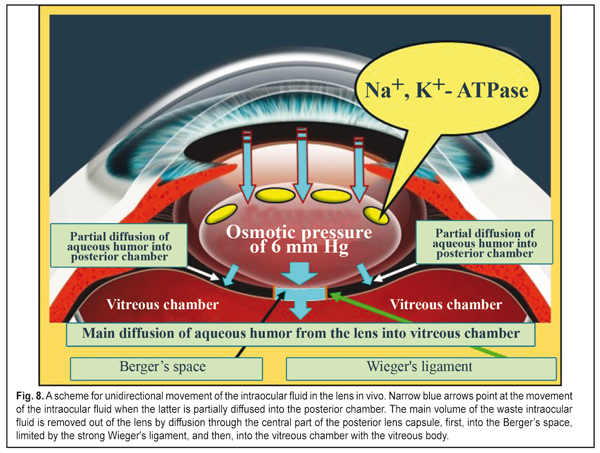
The intravital dye in the lens did not spread towards the anterior lens surface but only towards the posterior surface. And in the unfrozen enucleated eye, the dye moved both towards the anterior lens surface and into the vitreous chamber. This can be explained by the fact that Na+, K+- ATPase stopped functioning as a result of the freezing of the enucleated lenses and the dye was spread by diffusion in all directions. Thus, in intravital staining, the dye distribution in the lens cannot be described by simple diffusion and points at the presence of directed fluid transport from the anterior to posterior lens surface which is formed by the Na+, K+- ATPase transport system, located in the lens epithelium (a determining mechanism), and by the intralenticular pressure at different accommodation phases (an additional mechanism). To sum up the experimental study, we can have a generalized scheme for fluid circulation in the lens (Fig. 8) which is consistent with a mechanism of lens accommodation [9, 11, 14]. So, water-exchange processes in the lens are only partially connected with lens volume fluctuation at “near-far” accommodation moments. However, when looking at distance, the pressure in the flattened lens is minimal, so it is easier for the “fresh” fluid to flow into the lens through the anterior lens capsule.
The active Na+, K+- ATPase ionic transport system, located in the anterior lens capsule epithelium, is determining and facilitates the osmotic transport of the “fresh” fluid and its further one-direction diffusing from the anterior to posterior lens capsule surface not through the lens nucleus but along cortex laminae. In near vision, the inflow of the intraocular fluid additionally increases the intralenticular pressure up to 6 mm Hg by means of osmosis. When looking at close objects, the lens is maximally rounded and the pressure inside the lens is maximal, which facilitates a diffusing of the “waste” intraocular fluid through the posterior capsule into the vitreous chamber. The inhibitor in the incubatory media significantly decreased a lens weight gain since it, getting into the lens capsule together with external intraocular fluid, reduced the intensity of osmotic fluid transport in the lens. The incubatory solutions with inhibitors might decrease their ability not only to “plump” the fluid out of the lens but they also lost the ability to bind a balanced amount of the fluid. This made it possible to consider our experiments in this part of study sufficiently correct when mainly osmotic properties of the media inside the lens determined the pathways of fluid transport at different values of the intraocular pressure. The results given demonstrated that the mechanical mechanism of osmosis, likely, did not depend much on the accommodation phase and was able to transport into the lens such amount of the intraocular fluid that corresponded in norm to its ability to bind the fluid before a maximal possible physiological threshold level of the intralenticular pressure was achieved. In a live eye, the lens capsule at all accommodation phases was slightly or greatly stretched by the ciliary zonule, not by the fluid from inside, so the values of the intralenticular pressure did not reach the threshold level. When completely placing the lens into the incubatory solution with zero oncotic pressure, the lens volume was maximally increased mainly by the means of diffusion. This speaks to the fact that the lens can have an additional physiological excess (pool) of proteins for this fluid diffusion. And we believe that the presence of such “physyological pool” of the oncotic pressure in the lens is related to the need to provide the reliability of the lens nutrition even when the intraocular pressure is increased. It is as if in case, for instance, of benign ophthalmic hypertension associated with age-related growth of scleral rigidity, the IOP will grow in response, which will obstruct the outflow of the intraocular fluid. In this case, the physiological pool of “the oncotic pressure” inside the lens will make it possible to securely bind a great amount of the “fresh” intraocular fluid and to securely provide metabolic processes in a healthy lens by means of the lens epithelium even when there is an age-related increase of the intraocular pressure. Under-correction and mydriatic treatment of myopia lead to continued relaxation of the ciliary muscle and to the decreased intralenticular pressure [9, 11, 13]. This condition of the accommodative system is favorable for “plumping” the “fresh” intraocular fluid into the lens but it does not allow completely effectively to remove the “waste” intraocular fluid out of the lens. The obstructed flow of the “waste” intraocular fluid together with dissolved wastes out of the lens can lead to adverse effects. In fact, there appears disposition toward waste pollution of the lens, which can provoke cataract and presbyopia development. That is why, in ophthalmic practice, under-correction in the fight against adaptive myopia is not completely a physiologically adequate measure. Conclusions 1. The determining mechanism that normally providies the lens nutrition with the “fresh” intraocular fluid is active ionic-exchange transport with Na+, K+- ATPase located in the anterior lens capsule epithelium. 2. The changes in intralenticular pressur levels at different accommodation phases are only an additional mechanism, supporting the “waste” intraocular fluid removal out of the lens. 3. The oncotic pressure in the lens surrounding media must be 30 mm Hg and above to observe the constancy of the lens volume, i.e. the equality of the volume of the intraocular fluid coming into the lens by osmosis and coming out by diffusion. We can consider that the maintaining this threshold level of the oncotic pressure in the posterior aqueous humor in norm will make it possible to more thorough study the effect of accommodation on the genesis of refraction in various ametropias, to ground the importance to use these data for choosing a rational optical correction and slowing down cataract and presbyopic processes. 4. In long lasting stressing near work, the lens is maximally rounded and the intralenticular pressure is maximal. This partially decreases the ability of normal pumping the “fresh” intraocular fluid into the lens. A possible way for more successful fight against initial cataract and presbyopia is rational correction to normalize metabolic processes by avoiding the maximal lens rounding in long lasting comfort near work. That is why, in ophthalmic practice, over-correction in the fight against adaptive myopia is not completely a physiologically adequate measure. 5. When the ciliary muscle is relaxed, the volume of intraocular fluid production in ciliary processes is three times reduced. As a result, delivery volumes of the “fresh” intraocular fluid into the lens are decreased and metabolic processes in the lens are weakened. That is why, irrational correction can also contribute to accelerated development of cataract, especially in glaucoma eyes with myopia, since the flattened condition of the lens decreases the efficacy of operating mechanisms of “waste” intraocular fluid removal. That is why, in ophthalmic practice, under-correction in the fight against adaptive myopia is not completely a physiologically adequate measure as well. 6. When the ciliary muscle is relaxed, the lens, being flattened and set backwards, is actually pressed in the vitreous chamber, the pressure in which is increased. This leads to the fact that the flow of the “waste” intraocular fluid out of the lens into the vitreous chamber is difficult because of the increased pressure inside the vitreous chamber [12-14].
7. We formulated an important hypothesis that the level of the intralenticular pressure above 30 mm Hg can be a physiological barrier not only for normal lens nutrition (to facilitate cataract development) but it can be a certain barrier for normal functioning of fluid production in the ciliary processes in ophthalmic hypertension or glaucoma. Clinical trial for this hypothesis can greatly expand our ideas of a physiology-based upper level of the target intraocular pressure. References
|