J.ophthalmol.(Ukraine).2018;2:29-33.
|
https://doi.org/10.31288/oftalmolzh/2018/2/2933
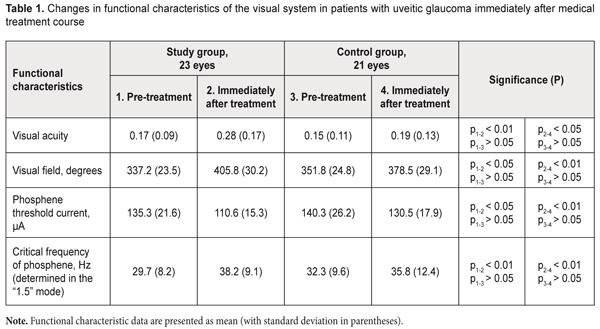
Efficacy of neuroprotective treatment including pyrimidine nucleotides in secondary uveitic glaucoma V. V. Savko Snr, Dr Sc (Med), V. V. Savko Jnr, Cand Sc (Med) Filatov Institute of Eye Disease and Tissue Therapy of NAMS of Ukraine; Odessa (Ukraine) E-mail: valchuk2001@ukr.net TO CITE THIS ARTICLE: Savko VV Snr, Savko VV Jnr. Efficacy of neuroprotective treatment including pyrimidine nucleotides in secondary uveitic glaucoma. J.ophthalmol.(Ukraine).2018;2:29-33. https://doi.org/10.31288/oftalmolzh/2018/2/2933 Background: Secondary glaucoma is found in 18% to 38% of patients with uveitis, and is one of the most severe uveitic complications. The glaucomatous process is characterized by the progressive optic nerve neuropathy. The lack of efficacy of conventional neuroprotective approaches urged us to search for novel medications capable of stabilizing glaucoma. Purpose: To investigate the efficacy of neuroprotective treatment with pyrimidine nucleotides for secondary uveitic glaucoma. Materials and Methods: Patients with secondary uveitic glaucoma were divided into the study group (23 patients; 23 eyes) and the control group (21 patients; 21 eyes). The multicomponent neuroprotective treatment of the study group (but not of the control group) included a direct neuroprotector, Nucleo CMP Forte® drug containing two pyrimidine nucleotides, cytidine 5'-monophosphate and uridine 5'- triphosphate. The group received 2 ml of the drug intramuscularly daily for 10 days, and, thereafter, 2 capsules orally twice daily for 20 days. Each patient underwent visual acuity measurement and perimetry. In addition, phosphene threshold current (PTC) and critical frequency of phosphene (CFP) were determined. Results: Although no improvements in functional characteristics of the visual system were observed in the control group, the use of the pyrimidine nucleotide-containing drug as a component of therapy for uveitic glaucoma contributed to a 39% improvement in visual acuity, 17% increase in total visual field, 22% decrease in PTC, and 23% increase in CFP compared with baseline. Our long-term findings evidence that glaucoma was stabilized with neuroprotective therapy including, in particular, Nucleo CMP Forte, with generally maintained peripapillary RNFL thickness. Conclusion: The use of the pyrimidine nucleotide-containing drug as a component of therapy for uveitic glaucoma promoted an improvement in visual function and contributed to stabilization of glaucoma over 6-8 months Keywords: secondary uveitic glaucoma, functional characteristics of the visual system, optical coherence tomography, pyrimidine nucleotides Introduction An important uveitis-related issue is that, is some cases, the course becomes chronic and the disease results in complications in different ocular structures, in spite of timely and best-available treatment [1]. Secondary glaucoma is found in 18% to 38% of patients with uveitis, and is one of the most severe uveitic complications [2-4]. The time course of increase in IOP in eyes with uveitis may vary. Trabecular edema and obstruction of the trabecular meshwork with inflammatory products can cause temporary increases in IOP (i.e., ocular hypertension) in acute uveitis. The IOP normalizes as signs of uveal inflammation (particularly, in the anterior chamber angle) subside when the inflammation is stopped. The condition is termed glaucoma secondary to uveitis (or secondary uveitic glaucoma) when IOP elevation persists due to progressive degenerative changes in the anterior chamber angle [3, 5]. Nesterov characterized uveitic glaucoma as open-angle, closed-angle and mixed forms. The open-angle form occurs due to trabecular degeneration, whereas the closed-angle form results from circular posterior synechiae with subsequent pupillary block. Chronic closed-angle glaucoma can develop even in the absence of pupillary block, with accumulation of inflammatory exudate in the anterior chamber angle, and with subsequent development of goniosynechiae. The mixed form is characterized by both trabecular damage and the development of goniosynechiae [3, 5]. It is currently believed that the glaucomatous process is characterized not only by elevated IOP and decreased aqueous outflow facility but also by the development of chronic progressive optic nerve neuropathy [6, 7]. In recent years, therapeutic measures aimed at preventing the neuronal apoptosis have become increasingly important. International multicentre studies have demonstrated that even effective reduction of IOP by pharmacological or surgical means does not always change the progressive course of functional glaucomatous decline, and these findings resulted in changes in the treatment strategy [8, 9]. Neuroprotection can be understood as a set of measures aimed at (1) reducing further degeneration of axons and (2) maintaining the structure of unaffected neurons of the optic nerve [7, 9]. Neuroprotection can be classified as direct or indirect. The action of direct neuroprotectors is aimed at stopping major ischemic damage directly in retinal neurons and optic nerve filaments [7, 9]. Indirect neuroprotection is aimed at blocking other damage factors, the main of which is elevated IOP [7]. In recent years, tissue-specific peptides (particularly, retinalamin) have become the most favored direct neuroprotectors for glaucomatous optic neuropathy among ophthalmologists [10]. In addition, the agent (1) contributes to normalization of protein synthesis and subsequent restoration of impaired fundus structures and vessel wall structures, (2) protects the vascular endothelium, and (3) regulates lipid peroxidation [10]. The use of retinalamin in patients with primary open glaucoma contributes to the improvement in visual acuity and electrical physiological parameters as well as to the increase in retinal nerve fiber thickness [8, 9, 11-13]. In spite of advances in treatment methods, currently, glaucoma therapy is still of low, if any efficacy and the disease is still a major cause of irreversible blindness in the world [14]. This situation urged us to search for new direct neuroprotection methods. We have had our attention drawn to Nucleo CMP Forte® drug (Ferrer Internacional SA, Spain) that contains two pyrimidine nucleotides, cytidine 5'-monophosphate (CMP) and uridine 5'- triphosphate (UTP). In contrast to other polypeptides that provide neuroprotection, this agent is aimed directly at the source of pathology. To the best of our knowledge, there is only one report on the use of Nucleo CMP Forte in patients with glaucoma [15]. Its major mechanism of action is a synthesis of phospholipids and glycolipids, the main components of the medullary sheath, which facilitates axonal regeneration after peripheral nerve injury. In addition, the mechanism is based on DNA chain elongation due to the addition of CMP and UTP units to the free nucleotide ends, which promotes a continuous DNA synthesis and impedes apoptosis in ganglion cells [16]. Nucleo CMP Forte has been widely used in the neurological treatment of (1) neuropathies of bone and joint, metabolic, or infectious origin, (2) geniculate, trigeminal or intercostal neuralgia and (3) lumbago. As Zhazykbaieva and colleagues [15] found that the agent improves optic nerve conduction in primary open-angle glaucoma, they recommended the agent for introduction into ophthalmological practice for this condition. However, to the best of our knowledge, there have been no reports on the application of the agent in uveitic glaucoma. The study purpose was to investigate the efficacy of neuroprotective treatment with pyrimidine nucleotides for secondary uveitic glaucoma. Materials and Methods Forty-four patients (44 eyes; age, 29 to 48 years; 26 men and 18 women) with the mixed form of secondary uveitic glaucoma were under our supervision. Of these, 11, 16, 14 and 3 patients had developed anterior uveitis lasting up to 24 years, 2 to 4 years, 5 to 7 years, and 8 to 10 years, respectively. In all patients, the inflammation was recurrent. Nine, 17, 13 and 5 patients developed secondary glaucoma 10 to 12 months, 1.5 to 2 years, 3 to 4 years, and 5 to 6 years, respectively, after the recurrence. In 12, 9 and 3 patients, the disease was found to be of streptococcal, tuberculous or chlamydial etiology, respectively. However, an etiology was not identified in the rest of patients. Throughout the observation and treatment period, all patients experienced sustained remission of anterior uveitis. In addition, the IOP was maintained in the range 20-26 mmHg with sinus trabeculectomy or hypotensive eye drops in 17 eyes (37%) and 27 eyes (63%), respectively. Patients were divided into the study group (23 patients; 23 eyes) and the control group (21 patients; 21 eyes). Both groups were comparable with regard to the sex (P = 0.734), age (P = 0.689), duration of primary disease (P = 0.562), visual acuity (P = 0.717; VA varied within the range 0.08-0.3), visual field (P = 0.692), and normalized IOP (P = 0.807). In addition, they were comparable in terms of the main clinical signs of the disease: the degree of ocular injection (P = 0.911), type of goniosynechia (P = 0.883), presence of pigmentation of the corneoscleral trabecula and Schlemm's canal (P = 0.329), presence of iris root atrophy (P = 0.426), type of posterior synechia (P = 0.767), state of the lens (P = 0.623), and vitreous opacities. Glaucomatous optic neuropathy was seen in all patients. Patients of both groups were treated with ATF (0.5 ml parabulbarly and 1.0 ml 1.5% intramuscularly, daily for 10 days), vitamin B6 (1.0 units intramuscularly, daily for 10 days), and pentoxifylline (400 mg orally, thrice daily for 30 days). In addition, patients of the study group were treated with Nucleo CMP Forte (2.0 ml intramuscularly daily for 10 days, then 2 capsules orally twice daily for 20 days). Each patient underwent visual acuity measurement, biomicroscopy, ophthalmoscopy, Maklakov tonometry, Goldmann lens gonioscopy, and assessment of total visual field through measurements at 8 principal meridians. In addition, phosphene threshold current (PTC) and critical frequency of phosphene (CFP) were determined using the FOSFEN-1 apparatus in the “1.5” mode. Peripapillary retinal nerve fiber layer (RNFL) thickness measurements were made with the SD-OCT Copernicus (Optopol, Zawiercie, Poland). Statistical analyses were conducted using Statistica 11.0 (StatSoft, Tulsa, OK, USA) software. Student’s t test was used for quantitative variables. Mann–Whitney U-test was used for intergroup comparisons of non-parametrically distributed variables (severity of the clinical signs). Results and Discussion Table 1 presents mean functional characteristics for both groups at baseline and immediately after the treatment course. The most substantial and significant changes in functional characteristics were observed only in patients of the study group, with the mean visual acuity improved from 0.17 (0.09) to 0.28 (0.16) (i.e., an improvement of 39%), total visual field extended from 337.2º (23.5) to 405.8º (30.2), PTC decreased 22%, from 135.3 (21.6) μA to 110.6 μA, and CFP increased 23%, from 29.7 (8.2) Hz to 38.2 (0.1) Hz. In the study and control groups, mean peripapillary RNFL thickness did not change after treatment and was 69.3 (16.8) µm and 73.2 (19.3) µm, respectively. The control group showed no statistically significant changes in functional characteristics after treatment.
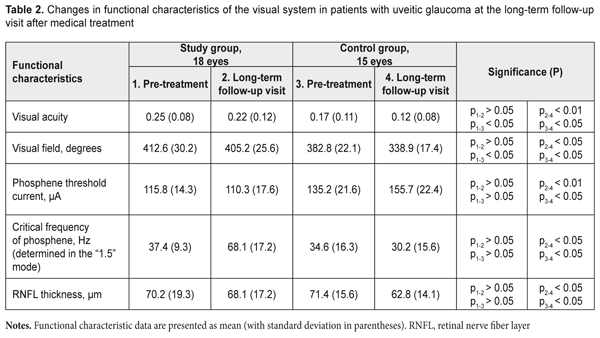
Table 2 presents long-term (6-8 months) outcomes following medicinal therapy in 18 patients of the study group and 15 patients of the control group. The study group showed no significant changes in functional characteristics (Table 2), evidencing stabilization of glaucoma. The functional characteristics, however, significantly worsened in the controls, with the mean visual acuity reduced 41%, from 0.17 (0.11) to 0.12 (0.008), total visual field reduced 13%, from 382.8º (22.1) to 338.9º (17.4), PTC increased 14%, from 135.2 (21.6) μA to 155.7 (22.4) μA, and CFP reduced 15%, from 34.6 (16.3) Hz to 30.2 (15.6) Hz. It should be noted that, in the study group, the mean peripapillary RNFL thickness was generally maintained over the supervision period, although reduced slightly (3%) and insignificantly from baseline (70.2 (19.3) µm) to the last follow-up visit (68.1 (17.2) µm, (P > 0.05)). The loss of RNFL thickness in the control group was more substantial and significant, with a 14% (8.6 µm) reduction from 71.4 (15.6) µm at baseline to 62.8 (14.1) µm at the last follow-up (P < 0.1).
Our findings evidence that glaucoma was stabilized with neuroprotective therapy including, in particular, Nucleo CMP Forte, in patients of the study group. Conclusion First, the use of Nucleo CMP Forte® drug containing two pyrimidine nucleotides, CMP and UTP, as one of the treatments in multicomponent therapy for uveitic glaucoma, contributed to a 39% improvement in visual acuity, 17% increase in total visual field, 22% decrease in PTC, and 23% increase in CFP compared with baseline. Second, our findings evidence that glaucoma was stabilized over a period of 6 to 8 months in patients of the study group, with improved optic nerve conductance (assessed by PTC data), improved papillomacular bundle function (assessed by CFP data), and generally maintained peripapillary RNFL thickness. Finally, the pyrimidine nucleotide-containing drug may be a promising component of a neuroprotective therapeutic approach for uveitic glaucoma. References
|