J.ophthalmol.(Ukraine).2018;6:65-70.
|
http://doi.org/10.31288/oftalmolzh201866570 Received: 25 October 2018; Published on-line: 31 December 2018 Acute vision loss in neurosurgical and neurological disorders V.A. Vasyuta, Dr Sc (Med); V.V. Biloshytskyi, Dr Sc (Med) Romodanov Neurosurgery Institute, National Academy of Medical Science of Ukraine; Kyiv (Ukraine) E-mail: Vasyuta.v@ukr.net TO CITE THIS ARTICLE: Vasyuta VA, Biloshytskyi VV. Acute vision loss in neurosurgical and neurological disorders. J.ophthalmol.(Ukraine).2018;6:65-70. http://doi.org/10.31288/oftalmolzh201866570
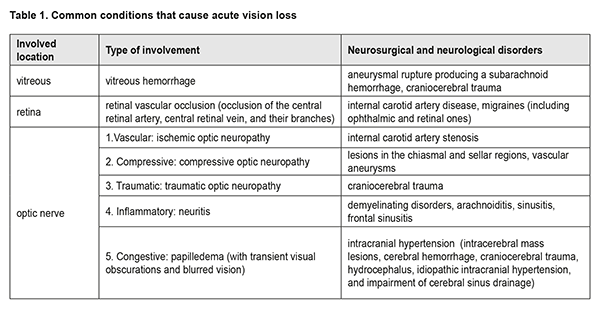
The evaluation and management of acute vision loss is a multidisciplinary problem. Aneurysmal rupture producing a subarachnoid hemorrhage, craniocerebral injury, internal carotid artery disease, migraines (including ophthalmic and retinal ones), and lesions in the chiasmal and sellar regions, intracranial hypertension and demyelinating disorders are common neurosurgical and neurological causes of this condition. Neuro-ophthalmological manifestations include retinal hemorrhage and vascular occlusion, ischemic, traumatic and compressive neuropathy, neuritis and papilledema. Early detection and adequate assessment of neuro-ophthalmological symptoms are of primary importance. Keywords: vision, acute loss, neurosurgical causes, neuro-ophthalmological manifestations, detection Visual pathway disorders are relevant to ophthalmology as well as neurology. The visual pathway is not only an essential portion of the visual system, but also a portion of the brain. Professor Ie. Zh. Tron The evaluation and management of acute vision loss (AVL) is an extremely difficult, multidisciplinary problem, and requires the close participation of ophthalmologists, neurosurgeons, neurologists and other specialists. The difficulty of the problem is that often AVL may be a manifestation of an eye disease or a sign of severe intracranial injury, and not only the patient’s sight, but also the patient’s life will depend on early diagnosis and adequate management strategy. Visual disorders affect quality of life and lead to depression [1]. The first doctor visited by a patient with AVL is the ophthalmologist. It is on the ophthalmologist depends whether or not the assessment of ocular symptoms is objective, the differential diagnosis is early, and adequate emergency care is provided to such a patient. Most cases of AVL are very serious and require active diagnosis and management. The condition results from a wide range of etiologies with various pathogenetic mechanisms; the most common are presented in Table 1.
It is important to distinguish between true sudden visual loss and the sudden realization that vision has been lost. Usually, the patient becomes aware of a sudden bilateral loss of vision immediately. Not infrequently, patients become aware of unilateral vision loss only when closing the fellow eye. Duration of vision loss is an important diagnostic criterion. Loss of vision may be transient (lasting from a few seconds to an hour), or prolonged (lasting more than an hour). Transient acute vision loss can be observed in amaurosis fugax, migraines, functional vision loss, and papilledema (with transient visual obscurations and blurred vision). Prolonged vision loss that develops suddenly is more common in central retinal artery (CRA) occlusion, central retinal vein (CRV) occlusion, vitreous hemorrhage, ischemic optic neuropathy, optic neuritis, and traumatic optic neuropathy and less common in pituitary apoplexy and atrophic papilledema associated with brain tumors. The blood supply to the visual system is closely associated with that to the brain. The ophthalmic artery (a branch of the internal carotid artery (ICA)) provides the blood supply to the prechiasmal visual pathway. The optic chiasm is supplied by the circle of Willis. The retrochiasmal visual pathway is supplied by branches of the ICA and the posterior and middle cerebral arteries [2, 3]. Vascular disorder is frequently accompanied by transient acute vision loss. Transient amaurosis is a symptom of impaired circulation to the ICA, whereas hemianopia with the exception of a small area around the fixation point is found in vertebral artery injury. The blood supply to the eye is mostly provided by branches of the ophthalmic artery, which is a branch of the ICA. This is why many patients with cerebral ischemia in the territory of the anterior circulation may present with complaints of visual disturbances [4]. The pathognomonic symptom of cerebral ischemia is ipsilateral visual changes. Bilateral visual impairment and visual field defects may occur in rare cases of bilateral internal carotid artery injury. Transient monocular vision loss (“amaurosis fugax”) is the most common ophthalmologic symptom of disease of the ICA [5, 6]. It is characterized by unilateral painless loss of vision lasting from a few seconds to a few minutes, and caused by transient ophthalmic artery spasm. The disorder is more common in patients aged > 50 years with diabetes mellitus, arterial hypertension, and hyperlipemia, i.e., all atherosclerotic risk factors. These patients may have ICA stenosis and history of transient ischemic attacks. Prolonged vision loss (either partial or total) of vascular etiology may result from occlusion of the central retinal artery or its branches in the presence of ICA stenosis. Ocular ischemic syndrome with the development of ischemic optic neuropathy is less common, not associated with injury to major vessels, and results from ischemic damage to small vessels of the optic nerve [7]. Partial or complete and total contralateral homonymous hemianopia is an important neuroophthalmological symptom of vascular injury to the brain and may appear as loss of vision in one eye only to the patient. The symptom occurs in occlusion of the branches of middle cerebral artery. In addition, it may result from occlusion of anterior choroidal arteries or their branches supplying the visual pathway and lateral geniculate bodies. Central retinal artery occlusion results in (usually unilateral) acute loss of vision or reduced visual acuity with visual field loss and characteristic fundus changes such as diffuse retinal discoloration, cherry red spot and arterial attenuation [8, 9]. In a few weeks, retinal vessels become refilled with blood and the retinal color returns to normal but optic disc discoloration ensues. Patients with stenosed carotid arteries have a high risk for the development of central retinal artery occlusion [8]. The prognosis for restoration of vision in these cases is doubtful. Conservative treatments including paracentesis of the anterior chamber ocular massage, aspirin and topical beta blockers have not been shown to alter visual outcome [10, 11]. Selective thrombolysis into the ophthalmic artery has been investigated as a promising treatment [12]. Page et al [13] have conducted the analysis of 236 patients treated with intra-arterial thrombolysis (IAT) and 255 patients treated with standard therapy for acute central retinal artery occlusion (CRAO). They found better changes in visual acuity and fields after treatment in former patients compared to latter. Their meta-analysis evaluating all controlled studies reporting IAT therapy for CRAO demonstrated a pooled OR significantly favoring IAT treatment. According to the American stroke association, a transient ischemic attack (TIA) is defined as transient neurologic dysfunction from, in particular, retinal ischemia, and patients with retinal ischemia should have brain neuroimaging, since TIA is an important predictor of stroke [1, 14, 15]. Having central retinal artery emboli increases the risk of stroke several fold, according to the Beaver Dam Eye Study involving as much as 4926 patients [16]. Occlusion or thrombosis of central retinal vein (CRV) is most common in elderly patients with atherosclerotic risk factors, in the presence of rheological changes in blood [17]. A patient with CRV occlusion typically presents with symptoms that include, in particular, a reduction in vision. In addition, the classic ophthalmoscopic picture shows diffuse retinal hemorrhage (“tomato ketchup”) and macular and optic disc edema [18]. Chiasmal strokes are rare, owing to the rich supply of collateral circulation provided by the Circle of Willis to the optic chiasm [19]. When chiasmal strokes do occur, patients experience acute onset bitemporal hemianopia and visual loss in a pattern resembling that seen in descending optic nerve atrophy. Binocular AVL can be observed in cortical blindness, hysterical blindness, and occlusion of branches of the posterior and middle cerebral arteries. Events of transient binocular visual loss may be a manifestation of vertebrobasilar ischemia, specifically ischemia in the territory of the posterior cerebral artery. Vision is always lost simultaneously in both eyes [20, 21]. Patients with transient binocular visual loss typically report “blurred vision” and/or “grey spot” in both eyes. The episodes last about a minute; longer episodes may be associated with a sensation of flashing before eyes. Fundus examination reveals moderately constricted vessels. Loss of vision secondary to central visual neuron injury is not infrequent. Bilateral ischemia or occipital infarction can cause cortical blindness [22]. Acute vision loss is not accompanied by an impaired pupil response and ophthalmoscopic changes. Patients with bilateral occipital infarction may develop Anton syndrome (associated agnosia of the cortical blindness) [23]. Migraine is a primary paroxysmal headache disorder affecting millions worldwide. Patients with migraine are prone to present with ophthalmological manifestations such as ocular pain, visual abnormalities and ophthalmoplegia. Approximately 15% to 20% of migraine sufferers experience visual auras that precede the onset of the headache. Most commonly, migraine aura is characterized by visual disturbances, e.g. scintillating scotoma that is perceived by the patient as a sudden short-term loss of vision with stereoscopic hallucinations, micropsia (Alice in Wonderland syndrome), and visual field loss. Migraine aura last for 5 to 60 minutes [24]. Migraine aura without headache (also known as acephalic migraine) occurs in 3-5% of migraine sufferers, most commonly, in elderly people. Visual abnormalities present in patients with acephalic migraine are the same as those in migraine aura with headache. The condition should be differentiated from transient ischemic attacks. Ophthalmic and retinal migraines have been given special attention among various types of migraine. Ophthalmic migraine (scintillating scotoma) was originally described by Doctor Hubert Airy in the 19th century. The pathogenesis involves circulatory insufficiency in the territory of the posterior cerebral artery. In some areas of the visual field, vision is transiently lost, and the patient has visual scintillations. Patients complain of scintillating scotomata, which are usually homonymous. At onset, a partial paracentral scotoma appears, and eventually expands to the visual periphery outwards; this may be accompanied by hallucinatory disturbances. Retinal migraine is characterized by transient (60 min or less in length), monocular episodes of visual disturbance (transient visual obscurations and blurred vision, scotoma or blindness), associated with migraine headache. These manifestations are caused by transient retinal ischemia. Headache may not be a pathognomonic sign of the disorder. The differential diagnosis includes ischemic transient monocular blindness and amaurosis fugax associated with internal carotid artery disease [25-27]. Vascular aneurysms may be accompanied by neuroopthalmological symptoms and acute vision loss. Clinical manifestations depend on the location and size of the vascular malformation. Aneurysms of the ophthalmic artery, anterior communicating artery, or carotid arteries may lead to optic neuropathy with visual field loss pattern similar to a chiasmal pattern of field loss [28]. Aneurysmal rupture producing a subarachnoid hemorrhage may be associated with retinal and vitreal hemorrhages in one or both eyes (so called “Terson syndrome”). The presumed mechanism is that of acute raised intracranial pressure with sudden elevation of ocular central venous pressure. Unless there is an associated retinal detachment, treatment is usually deferred and a vitrectomy is performed only if the hemorrhage does not resolve spontaneously [29]. Neuroopthalmic symptoms (such as visual loss from compressive or ischemic optic neuropathy and diplopia from ocular motor nerve compression) are most commonly revealed in paraclinoid aneurysms (aneurysms of ICA that arise near the origin of the ophthalmic artery). Schmidt et al presented a case series of eight patients who developed progressive visual loss immediately or shortly after uncomplicated coiling of a paraclinoid aneurysm. The authors believe that the visual loss was most likely caused by perianeurysmal inflammation, enlargement of the aneurysm in cases of incomplete clipping, and/or secondary ischemia of the affected site [28]. Lesions in the chiasmal and sellar regions have neuroophthalmic manifestations, chiasmal syndrome including changes in the visual field, decreased visual acuity and descending optic nerve atrophy. The most common presenting symptom in patients with chiasmal syndrome who attended a large ophthalmological institute was low visual acuity (54.8%) [31, 32]. The majority (65%) of chiasmal lesions have been reported to be caused by pituitary adenomas. Visual acuity commonly decreases gradually. Rare cases of sudden vision loss due to compression of the chiasm and optic nerves by giant tumors have been reported [33]. In pituitary apoplexy, fast extrasellular extension of the adenoma results in a sudden decrease or loss of vision, which is associated with headache, ophthalmoplegia and visual field loss [32, 34]. Tuberculum sella meningioma may compress the optic nerve in one or both eyes. The tumor commonly extends into the optic canal irrespective of lesion size [35, 36]. According to the study by Mahmoud et al [37], the tumor invaded the optic canal in 67% of patients with tuberculum sellae meningiomas. Neuroophthalmologic symptoms may suddenly develop in such cases. Commonly, sudden vision loss is accompanied by supraorbital pain and neurological and eye examination findings include afferent pupil defect and the visual field loss pattern similar to a chiasmal pattern of field loss. Changes in visually evoked potentials (VEP) at the onset of the process are not pathognomonic; increased VEP latency and decreased amplitude have been observed in long-lasting compression of the optic nerve. Retinal ganglion cell layer thinning is a major OCT finding in the retina and optic nerve of patients with tuberculum sellae meningiomas. Steroidal therapy may temporarily improve visual function in these patients. Studies in recent years have demonstrated the value of OCT imaging in quantitative assessment or nerve fiber loss in optic nerve compression and prediction of post-operative visual function improvement [37, 38]. The differential diagnosis includes optic neuritis, in which there are also decreased vision, pain posterior to the eye, and characteristic visual field changes but no ophthalmoscopic manifestations and there is a positive response to steroid therapy. There have been repoets on patients who had compressive optic neuropathy, but who were initially misdiagnosed as, and treated for optic neuritis [39, 40]. Ophthalmologists are often the first to see patients complaining of visual disturbances, and should bear in mind that even a small-sized parasellar meningioma can result in an acute visual disturbance due to tumor extension into the optic canal. Neuroimaging and immediate referral to the neurosurgeon are warranted in the absence of any apparent ocular loss of or reduction in vision. Elevated intracranial pressure may be accompanied by the development of papilledema. Possible conditions causing high intracranial pressure and papilledema include intracerebral mass lesions, cerebral hemorrhage, head trauma, hydrocephalus, impairment of cerebral sinus drainage, anomalies of the cranium, and idiopathic intracranial hypertension [41]. Transient visual obscurations and blurred vision may develop in the presence of apparent papilledema due to a short-term impairment in conduction of nerve fibers in the optic canal resulting from increased intracranial hypertension. Persistent optic disc swelling may result in severely reduced visual function. On fundus examination in the presence of optic disc swelling, one or both optic discs show signs of discoloration. The mechanism of vision loss in persistent optic disc swelling is associated with nerve fiber dysfunction and progressive nerve fiber loss with the secondary (post-papilledema) optic nerve atrophy. The development of complicated papilledema endangers sight and may involve sudden loss of vision. This is associated with two factors: elevated intracranial pressure and local influence of a basic pathological process on the nerve tissue involved in visual pathway formation. Damage to the papillomacular bundle and retinal hemorrhage may occur in these cases. Craniocerebral injury (CCI) is a cause of traumatic optic neuropathy [42, 43]. The condition is accompanied by impaired color vision and perimetric defects [44]. Decreased vision occurs due to either optic nerve tear within the optic canal or microfractures of the skull base leading to optic nerve ischemia, swelling and compression. Severe CCI may be accompanied by a total rupture of the optic nerve even in unaffected globe and in the absence of direct ocular trauma. The pathognomonic symptoms of this condition in the first few hours after traumatic event are sudden blindness in the affected eye, absence of direct pupil response to light, and presence of consensual light reflex. No fundus changes may be found in the first few days after traumatic event; post-traumatic optic nerve atrophy develops subsequently. Another possible cause of acute vision loss is intervention in any location of the body. Perioperative visual loss (POVL) [45] is a rare and devastating complication, and is more common after spine and cardiac surgeries (with the estimated incidences of 0.03-0.1% and 0.08%, respectively) than after other types of surgery. The causes of POVL are primarily retinal vascular occlusion (RVO) and ischemic optic neuropathy (ION), whereas cortical blindness is a rare cause of POVL. Painless visual loss and either afferent pupil defect or no direct pupil response to light are found in the first 24-48 hours after intervention. The pathogenetic risk factors are as follows: long intervention time (especially in spine surgery), decreased blood pressure, severe blood loss, altered venous hemodynamics, and presence of systemic vascular risk factors, including hypertension, diabetes, atherosclerosis, hyperlipidemia, and smoking history [46-48]. The prognosis for restoration of vision in these cases is always doubtful. Therefore, acute vision loss is common in patients with neurosurgical and neurological disorders. This is due to the anatomical relationship between the visual system and central nervous system. The structures along the visual system, from the retinal ganglion cell layer to the occipital cortex, are in intimate contact with brain structures. Direct compression, elevated intracranial pressure, local brain ischemia, and traumatic action are the basic pathogenetic mechanisms affecting the visual system. High proficiency in detecting and assessing neuroophthalmological symptoms and matching them with the neurological profile and performance of the patient are of primary importance as they allow saving vision and improving quality of life for patients.
References 1.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. doi: 10.1161/STR.0000000000000024. 2.Rowe FJ, Wright D, Brand D, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int. 2013; 2013: 719096. doi: [10.1155/2013/719096]. 3.Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. 4.Lamirel C, Newman NJ, Biousse V. Vascular Neuro-ophthalmology. Neurol Clin. 2010 Aug;28(3):701-27. doi: 10.1016/j.ncl.2010.03.009. 5.Biousse V, Trobe JD. Transient monocular visual loss. Am J Ophthalmol. 2005 Oct;140(4):717-21. 6.Fisher CM. Transient monocular blindness versus amaurosis fugax. Neurology. 1989 Dec;39(12):1622-4. 7.Luneak K, Newman NJ, Biousse V. Ischemic optic neuropathies. Neurologist. 2008 Nov;14(6):341-54. doi: 10.1097/NRL.0b013e318177394b. 8.Hayer SS, Podhajsky PA, Zimmerman MS. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009 Oct;116(10):1928-36. doi: 10.1016/j.ophtha.2009.03.006. 9.Hayer SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007 Mar;27(3):276-89. 10.Fiess A, Cal O, Kehrein S, Halstenberg S, Frisch I, Steinhorst UH. Anterior chamber paracentesis after central retinal artery occlusion: a tenable therapy? BMC Ophthalmol. 2014 Mar 10;14:28. doi: 10.1186/1471-2415-14-28. 11.Rudkin AK, Lee AW, Aldrich E, et al. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Exp Ophthalmol. 2010 Jul;38(5):496-501. doi: 10.1111/j.1442-9071.2010.02280.x. 12.Biousse V. Thrombolysis for acute central retinal artery occlusion: is it time? Am J Ophthalmol. 2008 Nov;146(5):631–4. doi: 10.1016/j.ajo.2008.07.025. 13.Page PS, Khattor NK, White AC, et al. Intra-arterial thrombolysis for acute central retinal artery occlusion: a systemic review and meta-analysis. Front Neurol. 2018 Feb 21;9:76. doi: 10.3389/fneur.2018.00076. 14.Biousse V, Newman NJ. Eye syndromes and the neuro-ophthalmology of stroke. Handb Clin Neurol. 2009;93:595-611. doi: 10.1016/S0072-9752(08)93029-3. 15.Pula JH, Yuen CA. Eyes and stroke: the visual aspects of cerebrovascular disease. Stroke Vasc Neurol. 017 Jul 6;2(4):210-220. doi: 10.1136/svn-2017-000079. 16.Klein R, Klein BE, Jensen SC, et al. Retinal emboli and stroke: the Beaver Dam Eye Study. Arch Ophthalmol. 1999 Aug;117(8):1063-8. 17.Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007 Mar;114(3):507-19, 524. 18.Shelton JB, Digre KB, Katz BJ, Warner JE, Quigley EP. Chiasmal stroke in patient with atrial fibrillation and complete occlusion of right internal carotid artery. J Neuroophthalmol. 2012 Jun;32(2):189. doi: 10.1097/WNO.0b013e3182491743. 19.Devuyst G, Bogousslavsky J, Meuli R, et al. Stroke or transient ischemic attacks with basilar artery stenosis or occlusion: clinical patterns and outcome. Arch Neurol. 2002 Apr;59(4):567-73. 20.Lauda F, Neugebauer H, Reiber L, et al. Acute silent brain infarction in Monocular Visual loss of ischemic origin. Cerebrovasc Dis. 2015;40(3-4):151-6. doi: 10.1159/000437274. 21.Glisson CC. Visual loss due to optic chiasm and retrochiasmal visual pathway lesions. Continuum (Minneap Minn). 2014 Aug;20(4 Neuro-ophthalmology):907-21. doi: 10.1212/01.CON.0000453312.37143.d2. 22.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache. 2005 Apr;45 Suppl 1:S3-S13. 23.Evans RW, Glosberg BM. Retinal migraine: migraine associated with monocular visual symptoms. Headache. 2008 Jan;48(1):142-5. doi: 10.1111/j.1526-4610.2007.00978.x. 24.Gloster BM, Solomon S, Friedman DI, Lipton RB. Retinal migraine reappraised. Cephalalgia. 2006 Nov;26(11):1275-86. 25.Pradhan S, Chung SM. Retinal, ophthalmic or ocular migraine. Curr Neurol Neurosci Rep. 2004 Sep;4(5):391-7. 26.Rodriguez-Catarino M, Frisen L, Wikholm G, et al. Internal carotid artery aneurysms, cranial nerve dysfunction and headache: the role of deformation and pulsation. Neuroradiology. 2003 Apr;45(4):236-40. 27.Biousse V, Newman NJ. Aneurysms and subarachnoid hemorrhage. Neurosurg Clin North Am. 1999 Oct;10(4):631-51. 28.Schmidt GW, Oster SF, Golnik KC, Tumialán LM, Biousse V, Turbin R, Prestigiacomo CJ, Miller NR. Isolated progressive visual loss after coiling of paraclinoid aneurysms. AJNR Am J Neuroradiol. 2007 Nov-Dec;28(10):1882-9. 29.Astorga-Corballo A, Serna-Ojeda JC, Camargo-Suarez MF. Chiasmal syndrome: clinical characteristics in patients attending an ophthalmological center. Saudi J Ophthalmol. 2007 Oсt-Dec; 31(4): 229–33. doi: 10.1016/j.sjopt.2017.08.004. 30.Wadud SA, Ahmed S, Choudhury N, Chowdhury D. Evaluation of ophthalmic manifestations in patients with intracranial tumours. Mymensingh Med J. 2014 Apr;23(2):268-71. 31.Sefi-Yardakul N. Visual findings as primary manifestations in patients with intracranial tumors. Int J Ophthalmol. 2015 Aug 18;8(4):800-3. doi: 10.3980/j.issn.2222-3959.2015.04.28. 32.Ogra S, Nichols AD, Stylli S, Kaye AH, Savino PJ, Danesh-Meyer HV. Visual acuity and pattern of visual field loss at presentation in pituitary adenoma. J Clin Neurosci. 2014 May;21(5):735-40. doi: 10.1016/j.jocn.2014.01.005. 33.Hayhurst C, Teo C. Tuberculum sella meningioma. Otolaryngol Clin North Am. 2011 Aug;44(4):953-63, viii-ix. doi: 10.1016/j.otc.2011.06.012. 34.Mahmoud M, Nader R, Al-Mefty O. Optic canal involvement in tuberculum sellae meningiomas: influence on approach, recurrence, and visual recovery. Neurosurgery. 2010 Sep;67(3 Suppl Operative):ons108-18; discussion ons118-9. doi: 10.1227/01.NEU.0000383153.75695.24. 35.Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011 Oct 31;52(11):8527-33. doi: 10.1167/iovs.11-8034. 36.Monteiro ML, Costa-Cunha LV, Cunha LP, Malta RF. Correlation between macular and retinal nerve fibre layer Fourier-domain OCT measurements and visual field loss in chiasmal compression. Eye (Lond). 2010 Aug;24(8):1382-90. doi: 10.1038/eye.2010.48. 37.Anderson D, Khalil MK. Meningioma and the ophthalmologist: diagnostic pitfalls. Can J Ophthalmol. 1981 Jan;16(1):10–5. 38.Lee AG, Lin DJ, Kaufman M, Golnik KC, Vaphiades MS, Eggenberger E. Atypical features prompting neuroimaging in acute optic neuropathy in adults. Can J Ophthalmol. 2000 Oct;35(6):325-30. 39.Rigi M, Almarzouqi SJ, Morgan ML, Lee AG. Papilledema: epidemiology, etiology, and clinical management. Eye Brain. 2015 Aug 17;7:47-57. doi: 10.2147/EB.S69174. 40.Hathiram BT, Khattar VS, Sonawane HP, Watve PJ. Traumatic optic neuropathy - our experience. Indian J Otolaryngol Head Neck Surg. 2010 Sep;62(3):229-35. doi: 10.1007/s12070-010-0072-y. 41.Chen YJ, Liang CM, Tai MC, et al. Longitudinal relationship between traumatic brain injury and the risk of incident optic neuropathy: A 10-year follow-up nationally representative Taiwan survey. Oncotarget. 2017 Sep 18;8(49):86924-86933. doi: 10.18632/oncotarget.21008. 42.Biousse V, Newman NJ. Diagnosis and clinical features of common optic neuropathies. Lancet Neurol. 2016 Dec;15(13):1355-1367. doi: 10.1016/S1474-4422(16)30237-X. 43.Roth S. Postoperative blindness, Anesthesia. 6th edition. Miller RD, ed. New York: Elsevier. 2015:2821–41. 44.Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012 Jan;116(1):15-24. doi: 10.1097/ALN.0b013e31823d012a. 45.Roth S. Perioperative visual loss: what do we know, what can we do? Br J Anaesth. 2009 Dec;103 Suppl 1:i31-40. doi: 10.1093/bja/aep295. 46.Kupersmith MJ, Berenstein A, Flamm E, et al. Neuroophthalmologic abnormalities and intravascular therapy of traumatic carotid cavernous fistulas. Ophthalmology. 1986 Jul;93(7):906-12. 47.Miller N. Carotid-Cavernous Sinus Fistulas. In: Miller N, Newman N, Biousse B, Kerrison J, editors. Walsh & Hoyt’s Clinical Neuro-Ophthalmology. 6. Philadelphia: Williams & Wilkins; 2005. pp. 1967–2168. 48.Chai Y, Yamazaki H, Kondo A, Oshitari T, Yamamoto S. Case of acute optic nerve compression caused by tuberculum sellae meningioma with optic canal involvement. Clin Ophthalmol. 2012;6:661-6. doi: 10.2147/OPTH.S30418.
|