J.ophthalmol.(Ukraine).2019;2:50-54.
|
http://doi.org/10.31288/oftalmolzh201925054 Received: 14 January 2019; Published-online: 24 April 2019 On selenium deficiency in Graves’ disease patients with autoimmune ophthalmopathy Yu.V. Buldygina, Cand Sc (Med); G.M. Terekhova, Cand Sc (Med); T.V. Fed’ko, Endocrinologist; V.M. Klochkova, Research Fellow; L.S. Strafun, Junior Research Fellow; I. I. Savosko, Endocrinologist; Z.G. Lysova, Endocrinologist Komisarenko Institute for Endocrinology and Metabolism of the NAMS of Ukraine; Kyiv (Ukraine) E-mail: Yuliya.buldygina@icloud.com TO CITE THIS ARTICLE: Buldygina YuV, Terekhova GM, Fed’ko TV, Klochkova VM, Strafun LS, Savosko II, Lysova Z.G. On selenium deficiency in Graves’ disease patients with autoimmune ophthalmopathy. J.ophthalmol.(Ukraine).2019;2:50-4. http://doi.org/10.31288/oftalmolzh201925054 Background: The role of selenium (Se) deficiency in the development of thyroid associated ophthalmopathy has been actively debated in the literature. The question is still debated in Ukraine due to the absence of data both on selenium deficiency in this country and blood selenium levels in Graves’ disease patients with autoimmune ophthalmopathology (AO). Purpose: To investigate blood selenium levels in Graves’ disease patients with AO. Materials and Methods: Fifty-seven Graves’ disease patients were included in the study. Of these, 44 had AO. Clinical severity of AO was classified using the NOSPECS. Of the 44 patients with AO, 16, 19 and 9 patients had class 2b, class 3a or 3b, and class 4a, respectively. All patients were euthyroids receiving antithyroid therapy (mean TSH level, 1.36 ± 0.4 mIU/L; reference TSH range, 0.4 mIU/L to 4.0 mIU/L). A Hitachi MPF-2A fluorescence spectrophotometer was used to determine blood selenium levels. A blood selenium level between 75 and 120 μg/l was considered normal, between 50 and 75 μg/l was moderate Se deficiency, and <200 μg/l considered severe Se deficiency. Results: Of the 57 patients of both groups, 31 (54%) were found to be severely selenium deficient. The mean blood selenium level in Graves’ disease patients with AO was 60.07±7.61 μg/l, i.e., substantially lower than the normal range. In addition, 34% of these patients had a normal blood selenium level, whereas 16% and 50%, moderate and severe Se deficiency, respectively. The mean blood selenium level in Graves’ disease patients without AO was 34.4±10.2 μg/l, i.e., also substantially lower than the normal range. In addition, 31% of these patients had a normal blood selenium level, whereas 69%, severe Se deficiency. Conclusion: First, irrespective of the presence or absence of AO, 54% of Graves’ disease patients were found to exhibit severe selenium deficiency. Second, the mean blood selenium level in Graves’ disease patients with AO was 60.07 ± 7.61 μg/l, and 50% of them exhibited severe selenium deficiency. In addition, the mean blood selenium level in patients with Graves’ disease only was 34.4 ± 10.2 μg/l, and 69% of them exhibited severe selenium deficiency. There was, however, no significant difference in blood selenium level between groups (Р > 0.05). Therefore, it may be assumed that selenium deficiency is not a factor affecting the development of AO in Graves’ disease. Finally, given that fact that 34% of Graves’ disease patients with AO exhibited no selenium deficiency, such patients should not be prescribed selenium agents without first checking their blood selenium levels. Keywords: Graves’ disease, autoimmune ophthalmopathy, selenium
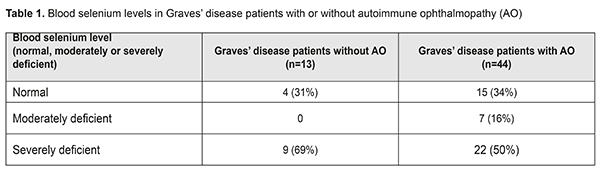
Introduction Essential trace elements, such as iodine, selenium, zinc, copper, and iron, and vitamin A, are known to be involved in the regulation and metabolism of endocrine systems. It has been demonstrated that selenium is a cofactor required to activate several human enzyme systems [1]. Selenium is integrated into the polypeptide chains as the 21st amino acid, selenocysteine and the proteins which contain selenocysteine are called selenoproteins (SPs). The key metabolic function of Se has therefore been attributed to its role in this enzymatic cofactor selenocysteine [2-4]. The importance of Se and selenoproteins in the treatment of thyroid gland (TG) has been increasingly acknowledged [5-7]. This is because they have a role in neutralization of the excess of H2O2 and reactive oxygen species that are overproduced in pathological hyperactivity and proportionately large quantity of Se are required to protect the thyroid gland from superoxide damage [8, 9]. In severe selenium deficiency, peroxide cleavage within the thyroid cells is diminished [10], and nutritional Se deficiency therefore leads to an increased rate of thyroid cell necrosis and invasion of macrophages and further increase in thyroid hormone levels in blood due to liberation of stored thyroid hormones [11-13]. Like iodine, Se also influences the size of the thyroid gland [14]. In addition, Se deficiency can exacerbate the effects of iodine deficiency and the same is true for vitamin A or iron deficiency [15, 16]. Se deficiency has shown to be a key environmental factor which is thought to precipitate autoimmune thyroid disease in several parts of the world deficient in soil Se [17]. The regulation and metabolism of thyroid hormones require a steady supply of Se, and environmental Se levels strongly correlate with serum Se levels [18, 19]. The two main autoimmune thyroid diseases which may be accompanied by autoimmune ophthalmopathy (AO) are Graves' disease (GD) which is the most common cause of thyrotoxicosis, and autoimmune thyroiditis (AIT) which is the most common cause of hypothyroidism. Ninety percent of patients with AO have GD and 10% suffer from AIT, and in the latter the ocular signs are often mild. Grave's disease is considered a classical autoimmune disease. The pathogenesis of GD is based on the production of unique IgG antibodies that bind to and activate the thyroid-stimulating hormone (TSH) receptor on the surface of thyroid follicular cells, which causes activation of adenylate cyclase and elevated intracellular cAMP level, leading to phosphorylation of protein kinase A and activation of various transcription factors. These processes result in increased iodine capture, increased production of thyroid peroxidase and thyroglobulin, and, eventually, in thyroid hyperfunction [20, 21]. In addition to thyrocytes, TSH receptors are also expressed in the orbital fibroblasts and preadipocytes and when bound by TSHR-Ab trigger a chronic inflammatory cascade resulting in the presence of infiltrate in the orbital tissue. This infiltrate is composed mostly of activated T cells producing cytokines (mainly IL-1, TNF-alpha, IFN-gamma) which, in turn, activate orbital fibroblast secretion of glycosaminoglycans, further inducing orbital fibrosis and edema [22, 23]. There is increasing evidence that antioxidant selenoenzymes are capable of modulating the course of autoimmune processes [24] in thyroid-associated ophthalmopathy. These mechanisms include 1) inhibitory effect of HLA–DR molecule expression on thyrocytes; 2) reductions of TSH receptor antibodies and antibodies to thyroid peroxidase; 3) prevention of dysregulation of cell -mediated immunity and B cell function; 4) neutralising reactive oxygen species and inhibition of redox control processes required for the activation, differentiation and action of lymphocytes, macrophages, neutrophils, natural killer cells involved in both acute and chronic orbital inflammation in AO; 5) inhibition of expression of pro-inflammatory cytokines and 6) inhibition of prostaglandin and leukotriene synthesis [25-27] . Selenium-containing agents for mild AO and mild autoimmune thyroid disease have become available and popular in recent years [28]. In the absence of reliable evidence supporting optimum dose and duration of selenium-containing agents, clinicians make decisions about a treatment regiment based largely on their personal preferences, but not on a universally recognized protocol. Treatment with a daily dose of 200 μg of Se agent for 6 months has become popular. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy was adopted [29]. These guidelines recommend that a 6-month selenium supplementation be given to patients with mild Graves’ orbitopathy (GO) of relatively short duration, because it improves eye manifestations and QoL and prevents GO progression to more severe forms. This scheme is based on the data on the reference dose, a 200-μg safe daily intake of Se supplement for normally nourished adults [30]. Selenium level is, however, rarely investigated in individuals enrolled in relevant studies before or during Se supplementation period. This situation is rather unsafe, since excessive Se intake has been reported to lead to toxic effects [31]. Excessive selenium intake can result in selenosis, manifested as changes in nails and hair (sometimes as severe as loss of nails and /or alopecia) [32, 33]. In addition, severe selenosis can result in impaired cognitive function, weakness, paralysis and even death. Given the risk for selenosis for individuals taking Se supplements for prolonged intervals, it is important to clarify whether all patients with GD associated with AO should be administered them, and what is an optimal supplementation regimen (dosage and frequency) for these patients.. The purpose of this study was to investigate blood selenium levels in patients with Graves’ disease complicated by autoimmune ophthalmopathy. Materials and Methods A Hitachi MPF-2A fluorescence spectrophotometer was used to determine blood selenium levels. The advantages of this apparatus include easy operation, adequate sensitivity (approximately 0.0004 μg/ml Se), low measurement error (2%) and a relatively low price. Fluoric determination of Se in blood is based on the reaction of selenium with 2,3-diaminonaphthalene to produce 4,5-benzopiazoselenol. The latter compound fluoresces under ultraviolet light and can be extracted from acid aqueous solution by toluene, decahydronaphthalene or cyclohexane. The latter is more suitable since 2,3-diaminonaphthalene is practically not soluble in it, and its complex with selenium can be easily extracted. The UV light absorbance maximum of 4,5-benzopiazoselenol in cyclohexane solution is at 378 nm and its fluorescence maximum is at 520 nm [34]. In the current study, a mixture of nitric acid with perchloric acid was heated to 100° С and used for isolation of selenium compounds from biological material. The Student t test was used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± SEM. Results and Discussion Fifty-seven patients (46 women and 11 men; age, 46.7±5.8 years) with Graves’ disease were included in this prospective study. Patients were divided into two groups based on the presence (group 1) or absence (group 2) of AO. Group 1 (GD plus AO) involved 44 patients (33 women and 11 men; age, 48.6 ± 4.37 years; range, 33 to 73 years) and group 2 (GD without AO), 13 patients (women only; age, 46.4 ± 5.5 years; range, 40 to 62 years). Clinical severity of AO was classified using the NOSPECS. Of the 44 patients with AO, 16, 19 and 9 patients had class 2b, class 3a (or 3b) and class 4a, respectively. Of the 13 patients of group 2, four had NOSPECS class 1a or class 1b. These four patients were not included in the group of patients with AO, since such changes are considered not manifestations of autoimmune ophthalopathy, but resulting from stimulation of the sympathetic nervous system by excessive hormones. A blood selenium level between 75 and 120 μg/l was considered normal, between 50 and 75 μg/l was moderate Se deficiency, and <200 μg/l considered severe Se deficiency. Of the 57 patients of both groups, 31 (54%) were found to be severely selenium deficient. The mean blood selenium level in patients with GD associated with AO was 60.07±7.61 μg/l (range, 1.5 to 142 μg/l), i.e., lower than the normal range. In addition, 34% of these patients had a normal blood selenium level, whereas 16% and 50%, moderate and severe Se deficiency, respectively. The mean blood selenium level in patients with GD without AO was 34.4±10.2 μg/l (range, 0.5 to 128 μg/l), i.e., also substantially lower than the normal range. In addition, 31% of these patients had a normal blood selenium level, whereas 69%, severe Se deficiency (Table).
No significant difference in blood selenium level was found between the groups (60.07±7.61 against 34.4±10.2 μg/l; Р>0.05). Therefore, irrespective of the presence or absence of AO, most patients with GD exhibited severe Se deficiency, which requires administration of selenium agents. These findings are in agreement with those reported by other studies on selenium deficiency in thyroid disorders in Ukraine, with severe selenium deficiency observed in patients with autoimmune thyroid disorders [17]. Patients with moderate and severe selenium deficiency are likely to differ in selenium treatment regimen required and duration of selenium treatment. The research is underway on this subject. Conclusion First, irrespective of the presence or absence of AO, 54% of patients with Graves’ disease were found to exhibit severe selenium deficiency. Second, the mean blood selenium level in patients with Graves’ disease associated with AO was 60.07 ± 7.61 μg/l, and 50% of these patients exhibited severe selenium deficiency. In addition, the mean blood selenium level in patients with Graves’ disease only was 34.4 ± 10.2 μg/l, and 69% of these patients exhibited severe selenium deficiency. There was, however, no significant difference in blood selenium level between groups (Р > 0.05). Therefore, it may be assumed that selenium deficiency is not a factor affecting the development of AO in Graves’ disease. Finally, given that fact that 34% of patients with Graves’ disease associated with AO exhibited no selenium deficiency, such patients should not be prescribed selenium agents without first checking their blood selenium levels. References 1.Przybylik-Mazurek E, Zagrodzki P, Kuźniarz-Rymarz S, Hubalewska-Dydejczyk A. Thyroid disorders-assessments of trace elements, clinical, and laboratory parameters. Biol Trace Elem Res. 2011 Jun;141(1-3):65-75. doi: 10.1007/s12011-010-8719-9. 2.Böck A, Forchhammer K, Heider J, Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991 Dec;16(12):463-7. 3.Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. 2011 Oct 18;8(3):160-71. 4.Hardy G, Hardy I, Manzanares W. Selenium supplementation in the critically ill. Nutr Clin Pract. 2012 Feb;27(1):21-33. 5.Rayman MP. Selenium and human health. Lancet. 2012 Mar 31;379(9822):1256-68. 6.Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008 Nov;52(11):1235-46. 7.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity and their role in human health. Antioxid Redox Signal. 2007 Jul;9(7):775-806. 8.Köhrle J. Pathophysiological relevance of selenium. J Endocrinol Invest. 2013 Nov;36(10 Suppl):1-7. 9.Köhrle J, Jakob F, Contempré B, Dumont JE. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005 Dec;26(7):944–84. 10.Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metab. 2002 Apr;87(4):1687–91. 11.Bhuyan AK, Sarma D, Saikia UK. Selenium and the thyroid: A close-knit connection. Indian J Endocrinol Metab. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S354-5. doi: 10.4103/2230-8210.104090. 12.Contempre B, Denef JF, Dumont JE, Many MC. Selenium deficiency aggravates the necrotizing effects of a high iodide dose in iodine deficient rats. Endocrinology. 1993 Apr;132(4):1866–8. 13.Contempre B, Le-Moine O, Dumont JE, Denef JF, Many MC. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta). Mol Cell Endocrinol. 1996 Nov 29;124(1-2):7-15. 14.Rasmussen LB, Schomburg L, Köhrle J, Pedersen IB, Hollenbach B, Hög A, et al. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur J Endocrinol. 2011 Apr;164(4):585-90. 15.Triggiani V, Tafaro E, Giagulli VA, Sabbà C, Resta F, Licchelli B, Guastamacchia E. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disord Drug Targets. 2009;18:277–94. 16.Doupis J, Stavrianos C, Saltiki K, Mantzou E, Mastrokostopoulos A, Philippou G, Alevizaki M. Thyroid volume, selenium levels and nutritional habits in a rural region in Albania. Hormones (Athens). 2009 Oct-Dec;8(4):296-302. 17.Goncharova OA. [Selenium and the thyroid gland (literature review and own research data)]. Endokrynologiya. 2014;19(2):149–55. Ukrainian. 18.Corvilain B, van Sande J, Laurent E, Dumont JE. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology. 1991 Feb;128(2):779-85. 19.Corvilain B, Laurent E, Lecomte M, Vansande J, Dumont JE. Role of the cyclic adenosine 3',5'-monophosphate and the phosphatidylinositol-Ca2+ cascades in mediating the effects of thyrotropin and iodide on hormone synthesis and secretion in human thyroid slices. J Clin Endocrinol Metab. 1994 Jul;79(1):152-9. 20.Girgis CM, Champion BL, Wall Jr. Current concepts in Graves' disease. Ther Adv Endocrinol Metab. 2011 Jun;2(3):135-44. 21.Ludgate M. Animal models of Graves' disease. Eur J Endocrinol. 2000 Jan;142(1):1-8. 22.Khalilzadeh O, Noshad S, Rashidi A, Amirzargar A. Graves' ophthalmopathy: a review of immunogenetics. Curr Genomics. 2011 Dec; 12(8): 564–75. 23.Weetman AP. Graves' disease. N Engl J Med. 2000 Oct 26;343(17):1236–48. 24.Taylor EW. Selenium and cellular immunity. Evidence that selenoproteins may be encoded in the +1 reading frame overlapping the human CD4, CD8, and HLA-DR genes. Biol Trace Elem Res. 1995 Aug-Sep;49(2-3):85-95. 25.Dhamasena A. Selenium supplementation in thyroid associated ophthalmopathy: an update. Int J Ophthalmol. 2014;7(2):365–75. 26.Rotondo Dottore G, Leo M, Casini G, Latrofa F, Cestari L, Sellari-Franceschini S, et al. Antioxidant actions of selenium in orbital fibroblasts: a basis for the effects of selenium in Graves’ Orbitopathy. Thyroid. 2017 Feb;27(2):271-278. 27.Calissendorff J, Mikulski EH, Larsen EH, Möller M. A prospective investigation of Graves’ disease and selenium: thyroid hormones, auto-antibodies and self-rated symptoms. Eur Thyroid J. 2015 Jun;4(2):93–8. 28.Marcocci C, Bartalena L. Role of oxidative stress and selenium in Graves′ hyperthyroidism and orbitopathy. J Endocrinol Invest. 2013 Nov;36(10 Suppl):15–20. 29.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9–26. 30.Patterson P, Levander OA. Naturally occurring selenium compounds in cancer chemoprevention trials: a workshop summary. Cancer Epidemiol Biomarkers Prev. 1997 Jan;6(1):63–9. 31.Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006 Autumn;36(4):409–20. 32.Yang GQ, Wang SZ, Zhou RH, Sun SZ. Endemic selenium intoxication of humans in China. Am J Clin Nutr. 1983 May;37(5):872–81. 33.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb 9;179(4073):588–90. 34.Nazarenko II, Kislova IV, Guseynov TM. [Fluorometric determination of selenium by 2,3-diaminonaphthalene in biological materials]. Zhurnal analiticheskoi khimii. 1975;30(4):733–7. Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|