J.ophthalmol.(Ukraine).2019;3:36-40.
|
Received: 21 May2019; Published on-line: 27 June 2019 http://doi.org/10.31288/oftalmolzh201933640 Efficacy of 810-nm diode laser transpupillary thermotherapy delivered using the methodology developed for small (T1) choroidal melanomas I.V. Tsukanova, Junior Research Associate; S.I. Poliakova, Dr Sc (Med); V.O. Naumenko, Dr Sc (Med), Prof.; N.V. Pasyechnikova, Assoc. Member of the NAMS of Ukraine, Dr Sc (Med), Prof. Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) E-mail: inna.sister@gmail.com TO CITE THIS ARTICLE: Tsukanova IV, Poliakova SI, Naumenko VO, Pasyechnikova NV. Efficacy of 810-nm diode laser transpupillary thermotherapy delivered using the methodology developed for small (T1) choroidal melanomas. J.ophthalmol.(Ukraine).2019;3:36-40. http://doi.org/10.31288/oftalmolzh201933640 Background: Diode laser transpupillary thermotherapy (TTT) used as a monotherapy may be a treatment of choice for small choroidal melanomas (CM). We developed the methodology for performing TTT, which involves four sessions delivered daily over four consecutive days (Information Bulletin No. 22, based on Pat. of Ukraine №102,890 issued 25.11.2015. Method for treatment of T1 choroidal melanoma), and which we have been using for the treatment of small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) for 15 years. Purpose: To assess the efficacy of 810-nm diode laser TTT delivered using the developed methodology in small tumors differing in magnitude of baseline characteristics. Materials and Methods: We assessed the efficacy of 810-nm diode laser TTT delivered using the developed methodology for the small (T1) CM (measuring ≤ 3 mm in protrusion into the vitreous and ≤ 12 mm in basal dimension). Eighty-eight patients (63 men (71.6%) and 25 women (28.4%); mean age, 55.9 (12.8) years (range, 23 to 82 years) with small (T1) CM were involved into the study. The right eye was affected in 41 cases (46.6%), and the left eye was affected in the other 47 cases (53.4%). At baseline, no patient had metastasis. Treatment outcome was categorized as positive if partial or complete scarring of the tumor site was observed or negative if tumor growth persisted. Results: Eighty-one patient (92.05%) reached a positive treatment outcome, and 7 (7.95%) had a negative treatment outcome. In addition, complete scarring or partial scarring was found in 81.5% and 18.5%, respectively, of those with a positive treatment outcome. The treatment outcome in patients even with small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) depended on tumor dimensions and tumor area at baseline. Patients with small CM of more than 2.18-mm protrusion into the vitreous, more than 8.45-mm basal dimension, and more than 66.6 mm2 tumor area were 2.80 times more likely to have a negative treatment outcome. Conclusion: We developed the methodology for performing TTT, which involves four sessions delivered daily over four consecutive days (Information Bulletin No. 22, based on Pat. of Ukraine №102,890 issued 25.11.2015. Method for treatment of T1 choroidal melanoma) for the treatment of small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension). The success rate of the TTT methodology developed for small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) was 92.05%. The efficacy of treatment of small (T1) CM depended on tumor parameters at baseline: patients with a small CM measuring < 2 mm in thickness, < 8 mm in basal dimension, and < 66 m2 in area were more likely to have a positive treatment outcome in the form of scarring of the tumor site in 81.5% of cases. Keywords: choroidal melanoma, diode laser transpupillary thermotherapy
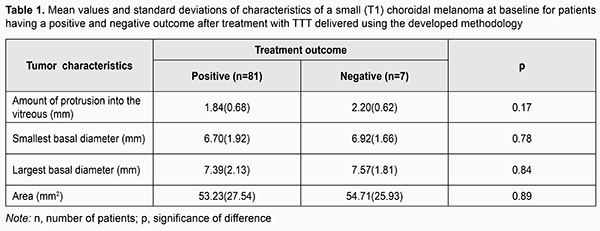
Introduction A small (T1) choroidal melanoma (CM) protruding 3.5-4.0 mm into the vitreous requires a special approach to the choice of treatment, since the neoplasm is in an early stage of its development, and there is a hope for better visual and survival prognosis [1-8]. Diode laser transpupillary thermotherapy (TTT) have been successfully utilized for treating CM since nineteen nineties [9-20], and allows to maintain visual function at a rather high level, while sparing healthy ocular tissues. TTT used as a monotherapy may be a treatment of choice for small choroidal melanomas. It is important that the procedure can be performed on an outpatient basis. We developed the methodology for performing TTT, which involves four sessions delivered daily over four consecutive days (Information Bulletin No. 22 based on Pat. of Ukraine №102,890 issued 25.11.2015. Method for treatment of T1 choroidal melanoma), and which we have been using for the treatment of small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) for 15 years. The purpose of the study was to investigate the efficacy of 810-nm diode laser transpupillary thermotherapy delivered using the developed methodology for CM differing in magnitude of baseline characteristics. Materials and Methods Eighty-eight patients (63 men (71.6%) and 25 women (28.4%); mean age, 55.9 (12.8) years (range, 23 to 82 years) with small (T1 according to the 2009 American Joint Committee on Cancer (AJCC)/ Union for International Cancer Control (UICC) staging system) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) treated at the Filatov Institute were involved into the study. The right eye was affected in 41 cases (46.6%), and the left eye was affected in the other 47 cases (53.4%). TTT was performed using a diode laser at 810nm (Iridis Quantel medical, France). The laser light was continuously delivered through one of the three lenses (the three-mirror Goldmann lens, Mainster PRP 165 or Mainster WF lens). The energy of the laser beam was gradually increased from 200 mW to 1800 mW to give the desired endpoint (either no color change or a subtle whitening of the tumor surfaces with no pain sensation) (Fig. 1). Laser spot size varied from 1.25 mm to 4 mm, depending on tumor basal dimension, proximity of functionally important structures (the macula and papillomacular bundle), and vessel location on the tumor surface. Overlapping treatment spots were applied from the periphery to the center to cover the entire tumor surface, including 1-2 mm of clinically normal tissue around the margin of the tumor. The exposure time was 60 seconds. Before each treatment session, the pupil was dilated with mydriatic drops, and one drop of topical alcaine 0.5% anesthetic was instilled into the conjunctival sac three times at interval of 2-3 minutes. After treatment sessions, a drop of non-steroidal anti-inflammatory drug (NSAID) was given five times daily over a week, whereas 1.0-ml parabulbar corticosteroid injection, oral NSAID and oral diuretic were administered daily for four days. The interval between follow-up visits was 2-3 months. If partial tumor regression was observed, the treatment course was repeated until complete scarring of the tumor site was observed. Each patient received between 2 to 9 TTT courses. The outcomes of treating small CM differing in baseline parameters (amount of protrusion into the vitreous; basal dimension (or diameter); and tumor area) using our methodology were subjected to analysis. Treatment outcome was categorized as positive if partial or complete scarring of the tumor site was observed by ophthalmoscopy of the fundus, ocular ultrasound, OCT or fluorescein angiography, or negative if tumor growth persisted. Follow-up duration varied from 12 to 180 months. At baseline, no patient had metastasis. Statistical analyses were conducted using Statistica 9 (StatSoft, Tulsa, OK, USA) software. Results and Discussion Overall, of small (T1) CM patients who received TTT treatment according to the developed methodology, 81 (92.05%) reached a positive treatment outcome, and 7 (7.95%) had a negative treatment outcome. In addition, complete scarring or partial scarring was found in 81.5% and 18.5%, respectively, of those with a positive treatment outcome. Table 1 presents mean values of tumor characteristics (amount of protrusion into the vitreous; basal dimension; and area) at baseline for patients having a positive and negative treatment outcome.
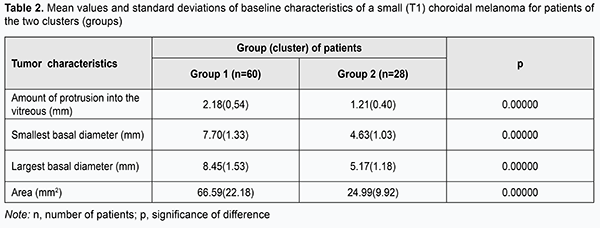
Although there was no statistically significant difference in tumor characteristics at baseline between patients having a positive and negative treatment outcome, characteristics for the latter patients were somewhat larger in magnitude than those for the former patients (amount of protrusion into the vitreous, minimum tumor diameter, maximum tumor diameter, and tumor area for patients having a negative treatment outcome were 0.36 mm, 0.22 mm, 0.18 mm and 1.48 mm2, respectively, larger than those for patients having a positive treatment outcome). Therefore, increased baseline tumor dimensions and area might be a potential unfavorable prognostic factor hampering clinical efficacy even in patients with small tumors. In an effort to decrease data scatter and create more homogeneous groups in terms of baseline tumor characteristics, we used an automatic clustering method to identify two clusters of patients based on the four baseline parameters, amount of protrusion into the vitreous, minimum tumor diameter, maximum tumor diameter, and tumor area. The first cluster (group 1) contained patients with larger tumors, and the second cluster (group 2), those with smaller tumors (Table 2).
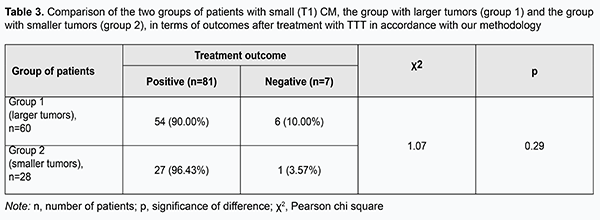
Choroidal melanomas in group 1 were statistically significantly larger in all the four parameters than those in group 2. Amount of protrusion into the vitreous, minimum tumor diameter, maximum tumor diameter, and tumor area for choroidal melanomas in group 1 were 0.97 mm, 3.07 mm, 3.28 mm and 41.60 mm2, respectively, larger than those for choroidal melanomas in group 2 (р = 0.000). Table 3 presents the comparison of the two groups of patients with small (T1) CM, the group with larger tumors (group 1) and the group with smaller tumors (group 2), in terms of treatment outcomes. Although in both groups positive outcomes outnumbered negative outcomes, it was found that the treatment outcome in patients even with small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) depended on tumor dimensions and tumor area at baseline. Patients with small CM of more than 2.18-mm protrusion into the vitreous, more than 8.45-mm basal dimension, and more than 66.6 mm2 tumor area were 2.80 times more likely to have a negative treatment outcome.
It has been reported that the success rate of TTT for small CM varied from 56% to 94% [1, 3-5, 9, 13, 16, 18]. We demonstrated a rather high success rate (92.05%) of our TTT methodology for small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension), allowing us to recommend the approach as a single therapy for such tumors. Early diagnosis of CM is important and can be made during routine ophthalmological check-ups (evaluation must be made with dilated pupils). Conclusion First, our methodology developed for performing TTT for small (T1) CM (measuring ≤ 3 mm in thickness and ≤ 12 mm in basal dimension) and involving four sessions delivered daily over four consecutive days demonstrated a success rate of 92.05%. Second, the efficacy of treatment of small (T1) CM depended on tumor parameters at baseline: patients with a small CM measuring < 2 mm in thickness, < 8 mm in basal dimension, and < 66 m2 in area were more likely to have a positive treatment outcome in the form of scarring of the tumor site in 81.5% of cases. References 1.Bulgakova ES. [Treatment of small choroidal melanomas with diode laser transpupillary thermotherapy]. [Abstract of Cand Sc (Med) Thesis]. Moscow: Helmholtz Research Institute for Eye Diseases; 2005. 25 p. Russian. 2.Bukhtiiarova NV. [Transpupillary thermotherapy in the multicomponent organ-sparing treatment of choroidal melanoma]. [Abstract of Cand Sc (Med) Thesis]. Cheliabinsk: Ural State Medical Academy of Additional Education; 2006. 22 p. Russian. 3.Iarovoi AA, Linnik LF, Magaramov DA, et al. [Diode laser transpupillary thermotherapy: the potential for the treatment of small choroidal melanomas]. Russkii meditsinskii zhurnal. 2004;5(2):77–81. Russian. 4.Ushenina LA. [Optimization of laser treatment for early choroidal melanoma]. [Cand Sc (Med) Thesis]. Cheliabinsk: Ural State Medical Academy of Additional Education; 2008. 114 p. Russian. 5.Damato B, Lecuona K. Conservation of Eyes with Choroidal Melanoma by a Multimodality Approach to Treatment: An audit of 1632 Patients. Ophthalmology. 2004 May;111(5):977-83. 6.Damato B. Developments in the management of uveal melanoma. Clin Exp Ophthalmol. 2004 Dec;32(6):639-47. 7.De Potter P, Levecq J. [Transpupillary thermotherapy in the treatment of choroid melanoma]. J Fr Ophtalmology. 2001 Nov;24(9):937-43. French. 8.De Potter P. [Treatment of intraocular melanoma: new concepts]. Bull Men Acad R Med Belg. 2003;158(1-2):103-11. French. 9.V.V. Volkov. [Laser treatment of intraocular melanoma]. Russkii meditsinskii zhurnal. 2001;2(1):3–7. Russian. 10.Mazunin IIu. [New treatment methods in pathologies of the choroid and retina including the use of subthreshold power od fiode infrared laser radiation]. Vestn Oftalmol. 2005 Jan-Feb;121(1):49-54. Review. Russian. 11.Currie ZI, Rennie IG, Talbot JF. Retinal vascular changes associated with transpupillary thermotherapy for choroidal melanomas. Retina. 2000;20(6):620-6. 12.De Potter P, Jamart J. Adjuvant indocyanine green in transpupillary thermotherapy for choroidal melanoma. Ophtalmology. 2003 Feb;110(2):406-13. 13.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of Metastatic Disease after Enrollment in the COMS Trials for Treatment of Choroidal Melanoma. Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123(12):1639-43. 14.Journee–de Korver JG, Oosterhuis JG, de Wolff–Rouendaal D, Kemme H. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Brit J Ophthal. 1997 Mar;81(3):234-9. 15.Kiratli H, Bilgiç S, Söylemezoglu F, Alaçal S. Peripheral subretinal pigment accumulation following transpupillary thermotherapy for choroidal melanoma. Ophthalmic Surg Lasers Imaging. 2008 Jan-Feb;39(1):60-2. 16.Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002 Feb;109(2):225-34. 17.Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995 Sep;102(9):1351-61. 18.Shields CL, Shields JA, Cater J, et al. Plaque radiotherapy for uveal melanoma: long–term visual outcome in 1106 consecutive patients. Arch Ophthalmol. 2000 Sep;118(9):1219-28. 19.Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol. 2002 Jun;13(3):135-41. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|