J.ophthalmol.(Ukraine).2019;4:69-74.
|
http://doi.org/10.31288/oftalmolzh201946974 Our current understanding of metastasis and the potential for predicting the course of head and neck squamous cell carcinoma D.I. Zabolotnyi, Dr Sc (Med), Prof., Acad. of the NAMS of Ukraine; E.V. Lukach, Dr Sc (Med), Prof.; M.B. Sambur, Dr Sc (Med) Prof. Kolomiichenko Institute of Otolaryngology, National Academy of Medical Sciences of Ukraine; Kyiv (Ukraine) E-mail: erwin@lukach.org TO CITE THIS ARTICLE: Zabolotnyi DI, Lukach EV, Sambur MB. Our current understanding of metastasis and the potential for predicting the course of head and neck squamous cell carcinoma. J.ophthalmol.(Ukraine).2019;4:69-74. http://doi.org/10.31288/oftalmolzh201946974
This review presents recent studies on the mechanisms of malignant neoplasm metastases, particularly, in head and neck squamous cell carcinoma (HNSCC). The involvement of circulating tumor cells (CTC) and cancer stem cells (CSC), and the role of epithelial-to-mesenchymal transition (EMT) and the reverse process, mesenchymal-to-epithelial transition (MET), in the establishment of metastases were noted. The major features of the EMT program are loss of E-cadherin-dependent intercellular adhesion of epithelial cells and increase in tumor cell motility and capacity for migration to and invasion of adjacent tissues and remote organs. Identifying the humoral and cell factors of epithelial cell microenvironment which induce and regulate the capacity of these cells for malignant transformation is important not only for understanding the mechanisms of oncogenesis, but also for considering whether it is possible to use them as predictive markers or therapeutic targets, which is important for clinical practice. The review provides data on the role of CTC in the course of HNSCC. It has been demonstrated that the presence of CTCs in patient blood correlated with a higher clinical stage of the disease, thereby evidencing the significant prognostic value of CTC blood levels in head and neck cancer patients and that the presence of CTCs could be used as a monitoring tool for tumor status of head and neck cancer, especially for the early detection of the tumor recurrence and progression, advanced disease and metastases, and assistance in therapeutic effect assessment. A current approach to identifying the character of and predicting the clinical course of malignant neoplasms is based on the use of the lymph node ratio (LNR) defined as the number of positive lymph nodes divided by the total number of lymph nodes excised. Numerous studies demonstrated that the LNR has a predictive value in head and neck squamous cell carcinoma, may be used as an additional prognostic parameter in combination with the revised TNM classification in HNSCC to predict the course of disease, select adjuvant therapy and control therapy efficacy. Keywords: head and neck squamous cell carcinoma, metastasis, circulating tumor cells, cancer stem cells, lymph node ratio (LNR)
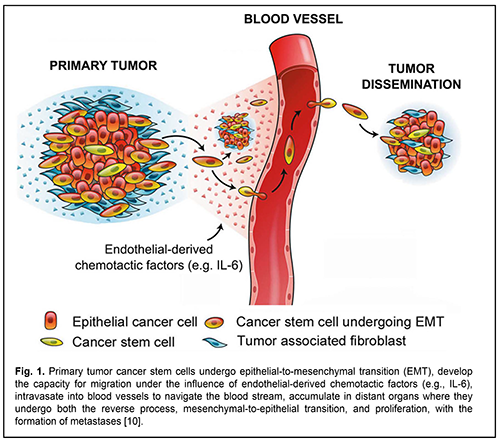
Malignant head and neck tumors are the 6th most common cause of morbidity and mortality due to cancer worldwide [1]. In Ukraine, they make up approximately 6% of the total human tumors [2], and, 45% of paranasal sinus and nasopharynx neoplasms grow into the orbit, making the diagnosis and treatment of this disorder an interdisciplinary issue [3]. Squamous cell carcinoma is the most common head and neck cancer. It is well known that the clinical course of and efficacy of treatment for malignant neoplasms are largely dependent on the development and spread of metastases, when steady-state cancer cells migrate through the bloodstream, extravasate, adapt to the new environment and evoke malignant growth at remote sites in the body, thereby aggravating the prognosis. New data on the role of stem cells in the development of malignant neoplasms have been presented in recent years. Our understanding of the mechanisms of oncogenesis has been improved by reports on the evidence of heterogeneity of primary tumors with respect to genetic, antigenic and other characteristics, providing a basis for positing that the development of malignant tumor metastases and recurrences is largely mediated by particular cell clones, cancer stem cells (CSC) [4-6]. The main features of CSC include high capacity for migration, resistance to radiation and drugs, differential mimicry, fusion with host cells, induction of neovascularization, and inhibition of both some immune responses and tumor growth [7-9]. Cancer stem cells differing in tumor types vary in surface markers. Although СD133, СD44, СD24 and epithelial specific antigen (ESA+) are considered major markers for CSC of solid tumors, site-specific proteins can be also identified. Malignant head and neck tumors can also express tumor markers [10, 11]. Recent studies have demonstrated that multipotent stem cells having a high tumor potential contribute to the formation of metastases of head and neck squamous cell carcinoma (HNSCC). Particularly, it has been shown that cancer stem cells reside in perivascular niches and are characterized by high aldehyde dehydrogenase (ALDH) activity and high CD44 expression (ALDHhighCD44high) in HNSCC [12]. A group of researchers from the Massachusetts Institute of Technology and Harvard Medical School developed a single-cell expression atlas of HNSCC primary tumors and metastases. In addition, they characterized the transition involving malignant and non-malignant cells within the tumor microenvironment which drives tumor spread. Cells expressing the partial epithelial-to-mesenchymal transition (p-EMT) spatially localized to the leading edge of primary tumors [1]. There is a growing evidence of the role of EMT in metastasis, with loss of E-cadherin-dependent cell-cell adhesion resulting in (a) a loss both of tumor cell capacity for close cell-cell contact and their adhesive properties and (b) increase in tumor cell motility and capacity for migration to and invasion of adjacent tissues and remote organs [13], where they regain their epithelial properties in a process called mesenchymal-to-epithelial transition (MET), which causes the formation of metastases [14, 15]. Inhibition of CDH1 expression leads to E-cadherin downregulation and is a factor influencing EMT [16]. A study by Beerling et al [17] demonstrated that the tumor cells that have lost E-cadherin expression and display a mesenchymal phenotype can migrate in vivo. In vitro cell line studies [20] and in vivo model studies [21, 22 ] have shown that EMT is induced by soluble growth factors, transforming growth factor alpha (TGF-?), interleukin-6 (IL-6) and transforming growth factor beta (TGF-?), and extracellular matrix molecules activating transcription factors Snail, Twist, Slug, ZEB1, ZEB2, Lef-1, etc. The endothelial cells secreting antiapoptotic protein Bcl-2 stimulate epithelial to mesenchimal transition and enhance the invasive capacity of CSC in patients with HNSCC. IL-6 enhances the capacity of CSC for migration and survival, and endothelial cell IL-6 secretion is a mechanism of tumor progression. Analysis of tissue microarrays generated from the invasive fronts of 77 HNSCC patients followed-up for up to 11 years [12] revealed that high expression of IL-6 receptor or co-receptor gp130 correlates with low HNSCC patient survival. Blockade of the IL-6 pathway with a humanized anti-IL-6R antibody (tocilizumab) inhibited endothelial cell-induced motility in vitro and decreased the fraction of cancer stem cells in vivo. Xenograft HNSCC tumors vascularized with IL-6-knockout endothelial cells exhibited slower tumor growth and smaller cancer stem cell fraction. That study showed that endothelial cell secreted IL-6 enhanced the motility and survival of cancer stem cells. These findings (a) provide grounds for asserting that, endothelial-derived IL-6 contributes to movement of cancer cells of the tumor and CSC through blood vessels to adjacent tissues where metastases develop, and (b) substantiate the potential for controlling metastasis process through administration of inhibitors of IL-6 production (Fig. 1) [12].
The proliferative activity and phenotypic characteristics of tumor cells (a) are regulated by humoral and cell factors of tumor microenvironment through epigenetic mechanisms, and (b) determine the metastazing capacity and drug resistance of a malignant neoplasm. On the other hand, the proteins involved in epithelial to mesenchymal transition have been identified, and may be used as predictive markers or therapeutic targets, which is important for clinical practice [23, 24]. In recent years, a concept has been proposed that the hybrid (or partial) epithelial-mesenchymal (E/M) phenotype, rather than either completely epithelial or mesenchymal phenotype is important for metastasis progression [25-27]. The metastasizing process is complex, with numerous cell types involved in its implementation, including the metastatic cells known as circulating tumor cells (CTCs) most of which are removed by immune defences, specifically, dendritic cells and macrophages [28]. The CTCs which evade destruction and removal by the immune mechanisms form metastatic tumors at distant sites [18]. Detection of CTS in patient blood samples is technically difficult due to a low level of these cells in the total population of blood cells. Thorough research has been conducted mainly in the areas of chemistry, materials science and bioengineering to develop a technology for identifying and isolating CTS and for determining their characteristics. Particularly, a research team at the University of California, Los Angeles, developed a unique concept of “NanoVelcro” cell-affnity substrates to immobilize CTCs, and the concept was utilized for developing cost-e?cient diagnostic platforms to monitor real-time disease progression [29]. Subsequently, Zhang et al [30] used a novel in vivo capture technique (CellCellector system) to detect CTCs in 24 HNSCC patients with primary or recurrent tumors (age, 47-81 years), demonstrating an important role of circulating tumor cells in the occurrence, progression and metastasis of HNSCC. In addition, the total capture rate of CTCs in patients with HNSCC before treatment was 70.8% (17/24), with 40% (2/5) for patients at I-? stage, and 78.9% (15/19) for patients at ?- ? stage, and was 0 in 9 healthy volunteers of control group [30]. Although various studies have assessed clinical and prognostic value of CTCs in patients with HNSCC, there is no unanimous agreement with regard to the prognostic value. Sun et al [31] systematically searched the studies with keywords in PubMed, MEDLINE, EMBASE, Science Citation Index Expanded and Cochrane Library (from inception to February 2017) to investigate the prognostic significance of CTCs in patients with HNSCC based on associations of positive CTCs were with poor overall survival, disease-free survival and progression-free survival. A total of 17 studies were included in the meta-analysis. Positive CTCs were significantly associated with poor overall survival, disease-free survival and progression-free survival. CTC-positive patients tended to have higher recurrence and regional lymph node metastasis rate and a more advanced tumor stage. The authors concluded that the meta-analysis had confirmed the significant prognostic value of CTCs in head and neck cancer patients. They believe that the presence of CTCs could be used as a monitoring tool for tumor status of head and neck cancer, especially for the early detection of the tumor recurrence and progression, advanced disease and the node metastasis [31]. In addition, it has been demonstrated that the dynamic determination of the number of CTCs in blood is an informative criterion of the efficacy of antitumor therapy for patients with HNSCC. Thus, a study by Lou et al [32] determined the numbers of CTC at baseline, two weeks after inductive chemotherapy and at the end of the treatment in twenty patients with local advanced HNSCC versus eight healthy cases as the negative control. In 75% of patients, CTC blood levels were significantly elevated at baseline. In 71.4% of the patients who received inductive chemotherapy, CTC blood levels substantially decreased after two weeks after inductive chemotherapy, and decreased nearly to background levels at the end of complete treatment. There was a correlation between the numbers of CTCs and age or N staging (P < 0.05). The authors concluded that CTCs have a high detection rate in the peripheral blood of patients with local advanced HNSCC, especially in patients ?60 years old and with ? N2 stage before treatment, and real-time detection of dynamic change of CTCs may assist to evaluate therapeutic effect [32]. Another area of recent research on identifying the treatment strategy and predicting treatment outcomes for patients with HNSCC has been investigation of the value of lymph node ratio (LNR) defined as the number of positive lymph nodes divided by the total number of lymph nodes excised. Particularly, the study by Sano et al [33] aiming to validate the concept of LNR in HNSCC included 63 patients with HNSCC who underwent resection of the primary tumor combined with neck dissection. It was found that LNR ? 0.068 was associated with poor overall survival (OS), progression-free survival (PFS) and locoregional recurrence-free survival (LFRS) after resection of the primary tumor combined with neck dissection in patients with HNSCC. Univariate and multivariate data analyses showed that LNR ? 0.068 was an independent prognostic factor for OS, PFS and LRFS. Both pathological T stage status (pT3 or 4) and ?3 positive LNs were also independent prognostic factors for PFS in patients with HNSCC in the univariate and multivariate analyses [33]. In a study evaluating the role of LNR as an additional predictive parameter to the 8th edition of AJCC TNM staging system [34], patients diagnosed with primary oropharyngeal squamous cell carcinoma (OPSCC) were enrolled, and of these, 137 underwent tumor resection with uni- or bilateral neck dissection. The proportion of human papilloma virus (HPV)-associated disease was 42%. Seventy per cent of patients presented with involved neck nodes. In p16-positive OPSCC, the rate of pN + cases was significantly increased compared to p16-negative OPSCC (86% vs. 58%, p = 0.007). Patients with LNR ? 10% had a significant better overall survival (OS) and disease-specific survival (DSS). However, when stratified for p16-status, LNR ? 10% had a significant impact on OS only for HPV-associated tumors, whereas LNR of ? 10% was not a significant predictor for better OS in p16-negative OPSCC. The authors concluded that the LNR with a cut-off value of 10% serves as an additional prognostic parameter in HPV-related OPSCC and may help to improve risk stratification in combination with the revised AJCC 8th edition TNM classification [34]. The role of LNR in the clinical practice for patients with HNSCC was also investigated in a study by Reinisch et al [35] aiming to evaluate to which degree LNR could be used as a more accurate predictor than TNM staging. The study demonstrated that LNE is an independent predictor of metastases at sites specific for the disease, correlates with the overall survival of patients with HNSCC, and is a more accurate predictor than TNM staging. It was found that impact of LNR on survival was significantly different even in patients with extracapsular spread. Patients without metastases (i.e., with pN0) had no survival benefit compared with patients with pN1 or higher with a LNR lower than 6 %. The authors concluded that LNR is a prognostic tool in patients with a lymph node status pN0-pN2b, and remained significant even in patients with extracapsular spread, contrary to TNM status. They believed that with LNR, stratification for high-risk patients (higher than 6 % LNR) could be evaluated easily, and suggested using LNR in the clinical routine. Therefore, numerous studies demonstrated the prognostic value of lymph node ratio (defined as the number of positive lymph nodes divided by the total number of lymph nodes excised) for predicting disease course of survival. The difference in values of characteristics taken into account in specific locations of squamous cell carcinoma by various studies ranges from 6.0 to 10.0%. Therefore, our review of studies reported in recent years gives evidence of a substantial progress in research on cancerogenesis mechanisms and malignant metastases, particularly with regard to head and neck squamous cell carcinoma. Our understanding of cancer stem cells and the role of EMT in tumor development and metastastic growth is not only of a theoretical value, but provides the basis for developing new approaches to cancer management, prediction of treatment efficacy, and creation of new agents and cancer vaccines for use in clinical practice as parts of multi-component cancer therapy regimens [4 , 13, 24, 36 ]. Targeted therapies for cancer are increasingly substantiated, and, in combination with other approaches, provide for a substantial improvement in efficacy of treatment of aggressive cancers. In addition, it is still to be determined whether developing metastasis prevention approaches based on inhibition of cancer cell migration would be effective [37-39 ]. Much attention is paid to developing novel effective diagnostic techniques, identifying the character of and predicting the clinical course of malignant neoplasms. Numerous studies demonstrated that the LNR defined as the number of positive lymph nodes divided by the total number of lymph nodes excised has a predictive value in head and neck squamous cell carcinoma, may be used as an additional prognostic parameter in combination with the revised TNM classification in HNSCC to predict the course of disease, select adjuvant therapy and control therapy efficacy. Further research with the use of novel technologies, preclinical models, and new techniques would enable more effective use of current knowledge on the mechanisms of oncogenesis at different phases of the onset and metastasis in the clinical practice. References 1.Puram S, Tirosh I, Parikh A, Patel A, Yizhak K, Gillespie S, et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell. 2017 Dec 14;171(7):1611-1624.e24. 2.Kolesnik OO, editor. [Cancer in Ukraine, 2016-2017. Incidence, mortality, indicators of the oncology service activity. Bulletin of the National Cancer Register]. Kyiv, 2018. Ukrainian. 3.Brovkina AF. [Diseases of the orbit]. Moscow: Meditsina; 1993. Russian. 4.Nassar D, Blanpain C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu Rev Pathol. 2016 May 23;11:47-76. 5.Meacham C, Morrison S. Tumour heterogeneity and cancer cell plasticity. Nature. 2013 Sep 19;501(7467):328-37. 6.Kreso A, Dick J. Evolution of the Cancer Stem Cell Model. Cell Stem Cell. 2014 Mar 6;14(3):275-91. 7.Lisianyi M, Grinevich Iu. [The role of stem cells in carcinogenesis and tumor immunotherapy]. Klinicheskaia Onkologiia. 2017;25(1):65–72. Russian. 8.Zheng X, Carstens J, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015 Nov 26;527(7579):525-530. 9.Fischer K, Durrans A, Lee S, Sheng J, Li F, Wong S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015 Nov 26;527(7579):472-6. Epub 2015 Nov 11. 10.Grinevich Iu. [Ways of development of immunotherapy in oncology. Review of studies performed at the National Cancer Institute]. Klinicheskaia Onkologiia. 2016;21(1):76–80. Russian. 11.Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014 Mar 11;14:173. 12.Kim H, Chen Y, N?r F, Warner K, Andrews A, Wagner V, et al. Endothelial-derived interleukin-6 induces cancer stem cell motility by generating a chemotactic gradient towards blood vessels. Oncotarget. 2017 Nov 1;8(59):100339-100352. 13.Kudriavets IuJ, Bezdenezhnykh NO, Semesiuk NI, Zhilchuk AV, Likhova OO, Kovaliova OA, et al. [Microenvironment of tumor cells as a source of modifiers of epithelial-mesenchymal transition and target for personalized anticancer therapy]. Oncologiia. 2016;18(4), 269-76. Ukrainian. 14.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016 Jun 30;166(1):21-45. 15.Jolly M, Ware K, Gilja S, Somarelli J, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017 Jul;11(7):755-769. 16.Gall T, Frampton AE. Gene of the month: E-cadherin (CDH1). J Clin Pathol. 2013 Nov;66(11):928-32. 17.Beerling E, Seinstra D, de Wit E, Kester L, van der Velden D, Maynard C, et al. Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity. Cell Rep. 2016 Mar 15;14(10):2281-8. 18.Tsubakihara Y, Moustakas A.. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor ?. Int J Mol Sci. 2018 Nov 20;19(11). pii: E3672. 19.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-?-induced epithelial–mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. 20.Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017 Jan;11(1):28-39. 21.Tran HD, Luitel K, Kim M, Zhang K, Longmore GD, Tran DD. Transient SNAIL1 Expression Is Necessary for Metastatic Competence in Breast Cancer. Cancer Res. 2014 Nov 1;74(21):6330-40. 22.Ye X, Tam W, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015 Sep 10;525(7568):256-60. 23.Satelli A, Mitra A, Brownlee Z, Xia X, Bellister S, Overman M, et al. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res. 2015 Feb 15;21(4):899-906. 24.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science. 2013 Feb 1;339(6119):580-4. 25.Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: Present and future in clinical oncology. Molecular Oncology. Mol Oncol. 2017 Jul;11(7):718-738. 26.Saitoh M. Involvement of partial EMT in cancer progression. J Biochem. 2018 Oct 1;164(4):257-264. 27.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018 Feb;18(2):128-134. 28.Massagu? J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016 Jan 21;529(7586):298-306. 29.Lin M, Chen J, Lu YT, Zhang Y, Song J, Hou S, et al. Nanostructure Embedded Microchips for Detection, Isolation, and Characterization of Circulating Tumor Cells. Acc Chem Res. 2014 Oct 21;47(10):2941-50. 30.Zhang H, Gong S, Liu Y, Liang L, He S, Zhang Q, et al. [The significance of circulating tumor cells in head and neck squamous cell carcinoma: a preliminary study]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018 Jan 7;53(1):39-44. 31.Sun T, Zou K, Yuan Z, Yang C, Lin X, Xiong B. Clinicopathological and prognostic significance of circulating tumor cells in patients with head and neck cancer: a meta-analysis. Oncotargets Ther. 2017 Aug 4;10:3907-3916. 32.Lou J, Guo L, Zheng WH, Zhao J, Zhao JQ, Liang Z. [Peripheral blood circulating tumor cells in local advanced head and neck squamous cell carcinoma]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Nov 7;52(11):824-829. 33.Sano D, Yabuki K, Takahashi H, Arai Y, Chiba Y, Tanabe T, et al. Lymph node ratio as a prognostic factor for survival in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx. 2018 Aug;45(4):846-853. 34.Jacobi C, Rauch J, Hagemann J, Lautz T, Reiter M, Baumeister P. Prognostic value of the lymph node ratio in oropharyngeal carcinoma stratified for HPV-status. Eur Arch Otorhinolaryngol. 2018 Feb;275(2):515-524. 35.Reinisch S, Kruse A, Bredell M, L?bbers HT, Gander T, Lanzer M. Is Lymph-node Ratio a Superior Predictor than Lymph Node Status for Recurrence-free and Overall Survival in Patients with Head and Neck Squamous Cell Carcinoma? Ann Surg Oncol. 2014 Jun;21(6):1912-8. 36.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014 Jul 10;511(7508):246-50. 37.Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, Thiery JP. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med. 2014 Oct;6(10):1279-93. 38.Marcucci F, Stassi G, De Maria R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016 May;15(5):311-25. 39.Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018 Apr;556(7702):463-468. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|