J.ophthalmol.(Ukraine).2019;4:43-48.
|
http://doi.org/10.31288/oftalmolzh201944348 Comparing structural changes in the rabbit’s uveal tract after the use of a radiowave surgery unit versus a cutting tool O.V. Khomiakova, Ophthalmologist; V.V. Vit, Dr Sc (Med), Prof.; A.P. Maletskyi, Dr Sc (Med) SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) E-mail: maletskiy@filatov.com.ua TO CITE THIS ARTICLE: Khomiakova OV, Vit VV, Maletskyi AP. Comparing structural changes in the rabbit’s uveal tract after the use of a radiowave surgery unit versus a cutting tool. J.ophthalmol.(Ukraine).2019;4:43-48. http://doi.org/10.31288/oftalmolzh201944348
Background: Organ-saving treatment strategies for uveal melanoma are still the most common therapeutic approach. Since there is insufficient experience of the use of radiowave surgery technologies, further research is required regarding (a) the effect of a radiowave surgery unit on histology of the tumor and healthy ocular tissues and (b) the changes in post-operative wound healing after implementation of various intervention techniques. Purpose: To assess the response of the sclera and uveal tract to the use of a radiowave surgery unit versus a cutting tool in animals. Materials and Methods: The studies were performed on rabbits taken from the vivarium of the Filatov Institute. A total of 12 Chinchilla adult male rabbits (age, 4–6 months; 12 eyes) were used in the study. Six rabbits of the experimental group (Group 1) underwent monolateral excision of ocular structures (the iris, ciliary body and choroid) with a 4.0-MHz radiowave surgery unit (Surgitron, Ellman International) set on monopolar mode. Results: Histomorphological studies of the ocular tissues (sclera, iris, ciliary body and choroid) in experimental rabbits demonstrated that after surgery with the use of a radiowave surgery unit, necrotic and degenerative changes were observed in the ocular tissues adjacent to the wound canal which had been preserved intraoperative, and these changes expanded to the retina. The changes in the tissues after the use of conventional cutting tools were less apparent. Conclusion: After surgery with the use of a radiowave surgery unit, necrotic and degenerative changes developed in the ocular tissues (ciliary body, scleral and sunconjunctival tissue fragments) adjacent to the wound canal which had been preserved intraoperative. The above changes were maintained, tending to become more severe over 30 days. Keywords: uvea, radiowave surgery, experimental model, histomorphological changes
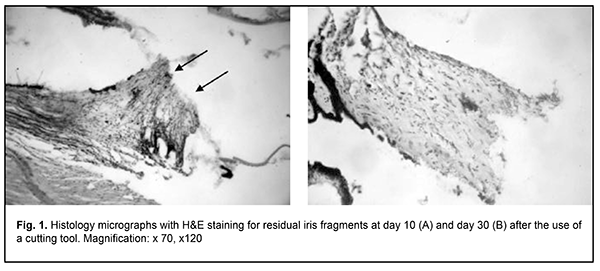
Introduction Intraocular melanoma is a highly malignant and rare tumor making up less than 1% of all cancers. In addition, it accounts for 12% of all melanomas and 80-87% of all intraocular tumors, whereas retinoblastoma accounts for another 11-18% of all intraocular tumors and is most commonly found in early childhood [1, 2]. In the general population uveal melanoma (UM) is uncommon with an incidence of 8–13.5 cases per million population per years [3]. In spite of advances in the diagnosis and management of UM, survival is poor and five-year and ten-year mortality rates among UM patients treated with enucleation are of 16.5% and 58%, respectively [4]. The prognosis is usually poor in patients with UM with extrascleral extension, with a ten-year mortality rate up to 69-73% [4]. Since the prognosis for survival largely depends on the on the stage at which the tumour is detected, early UM diagnosis is crucial. The peak age of diagnosis is 55 years [5]. In uveal melanoma, the iris, ciliary body and choroid may be affected; therefore, a differential approach to the management of this tumor is important. Anterior uveal melanoma accounts for 28-35% of all uveal melanomas. Organ-saving treatment strategies for patients with UM have been actively developed in recent years [4, 6-13], are aimed at saving the eye and its functions (with a treatment success rate of 41-52%), and provide for a substantially improved survival prognosis in these patients. Thus, five-year and ten-year survival rates among UM patients undergoing an organ-saving treatment were as high as 85.1-94% and up to 90%, respectively [4], and were substantially higher than those for patients treated with enucleation. Although recent advances in ophthalmic microsurgery significantly improved the potential for removing melanomas in the iris, ciliary body and choroid, the rates of intra- and postoperative complications for these patients are still high and affect treatment outcomes. Various authors (Linnik, 2000; J.Garcia-Arumi et al, 2001; etc.) reported that the main intraoperative complications were expulsive hemorrhage, choroidal hemorrhage, and partial vitreous hemorrhage, whereas the postoperative complications included cataract in 40%, retinal detachment in 16%, epiretinal macular proliferation in 8%, submacular hemorrhage in 4%, and hypotony resulting in ocular subatrophy. Tumor resection with radiosurgery enables simultaneous tissue resection and vessel coagulation; we believe it may be used for reducing the rates of intra- and postoperative complications for uveal melanoma patients. There have been no reports on this topic, to our best knowledge. The purpose of the study was to assess the pathomorphological ocular structural changes after radiosurgical intervention versus the use of a cutting tool in animals. Materials and Methods The studies were performed on rabbits taken from the vivarium of the Filatov Institute. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006, General Ethical Principles of Animal Experiments (First National Congress on Bioethics, Ukraine, Kyiv, 2001) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. A total of 12 Chinchilla adult male rabbits (age, 5–6 months and weight, 2–3 kg) were used in the study. The animals were housed and bred under similar conditions. Animals underwent surgery under general anesthesia with 0.1% pentobarbital sodium (1 ml/kg, intramuscularly). Six rabbits of the experimental group (Group 1) underwent monolateral excision of ocular structures (the iris, ciliary body and choroid) with a 4.0-MHz radiowave surgery unit (Surgitron, Ellman International) set on monopolar mode. Six rabbits of the control group (Group 2) underwent monolateral excision of ocular structures (the iris, ciliary body and choroid) with the use of conventional cutting tools. Air embolism was used for euthanasia as per the Helsinki declaration [1]. The animals were randomized for euthanasia at either day 10 or day 30. Enucleated globes were embedded in paraf?n. Serial sections were cut, deparaf?nized, and stained with hematoxylin and eosin (H&E). Histologic evaluations were performed by light microscopy (JonaMed, Germany). Non-parametric statistics was used for statistical analysis with Statistica 10.0 software (StatSoft, Inc., Tulsa, OK) [7]. Clinical signs (conjunctival chemosis, condition of sutures, presence or absence of discharge, anterior chamber hemorrhage, and vitreous hemorrhage) were scored daily from day 1 to day 10, and, subsequently, once in five days using the following score system: Conjunctival chemosis (0, no chemosis; 1, chemosis at the site of postoperative sutures; 2, chemosis at the site of postoperative sutures and adjacent conjunctiva; 3, marked conjunctival chemosis and orbital soft tissue swelling); Condition of sutures (0, no suture dehiscence; 1, individual sites of up to 1.0 mm dehiscence of the suture line; 2, sites of 1.0-5.0 mm dehiscence of the suture line; 3, dehiscence along the suture line); conjunctival discharge (0, no conjunctival discharge; 1, some sanious discharge in the conjunctival sac and at lid margins; 2, some serosanious discharge in the conjunctival sac, at lid margins and at the orbital site; 3, marked serosanious discharge in the conjunctival sac, at lid margins, at the orbital site and beyond the orbit); Anterior chamber hemorrhage and vitreous hemorrhage (0, no anterior chamber or vitreous hemorrhage; 2, hemorrhage in one third of the anterior chamber; 3, hemorrhage in half of the anterior chamber; 3, presence of a total hyphema and/or total vitreous hemorrhage). Results After surgery with the use of a cutting tool, all operated animals exhibited marked conjunctival chemosis at day 1, and suture condition was satisfactory along the follow-up period. In addition, in the first five postoperative days, there was a marked conjunctival discharge, which subsided at days 10 and 11. Moreover, there was an anterior chamber and vitreous hemorrhage intraoperative and in the first 5-10 days. After surgery with a radiowave surgery unit (Surgitron, Ellman International), operated animals exhibited marked conjunctival chemosis which subsided in 2 to 3 days. In addition, suture condition was satisfactory along the follow-up period, there was an insubstantial conjunctival discharge, and no anterior chamber or vitreous hemorrhage was observed. After iris and ciliary body dissection with the use of a cutting tool, pathohistological findings included similar changes in the ocular structures with the presence of clear wound canal margins irrespective of the structures through which the tool has passed. We observed no marked structural changes in survived tissues (Figs 1 a, b).
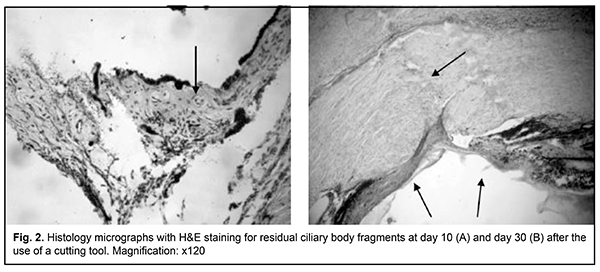
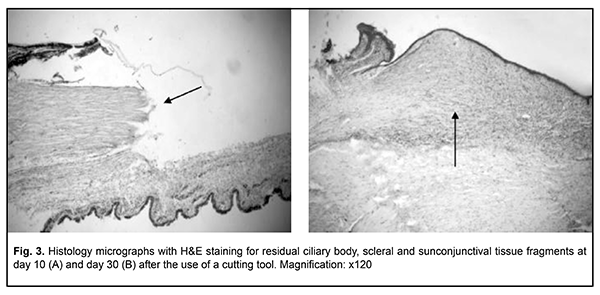
Although only a clear dissection margin and some lymphoid stromal infiltration were noted at day 10, stromal edema without inflammatory infiltration or signs of stromal fibrosis was observed at day 30. There was evidence of detritus developing from mechanical tissue damage in the wound canal. There were similar changes in ciliary body dissection (Fig. 2) and in ciliary body and scleral site dissection (Fig. 3).
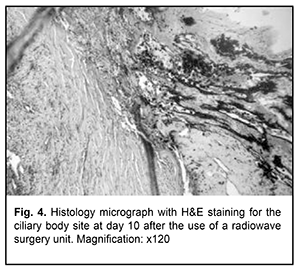
Although a clear dissection margin and no inflammatory response was noted at day 10, fibrosis of the structural tissue elements that had sustained intraoperative damage was observed at the end of month 1. Fibrous tissue was located mostly subconjunctivally, and in 2 cases (66.7%) it was hyperplastic. It is noteworthy that, the conjunctival epithelium covered the wound surface as early as day 7, that is, no substantial degenerative or inflammatory changes in the survived tissues were observed a week after surgery. However, massive subconjunctival tissue fibrosis was noted at day 30 (Fig. 3b). We found that the use of a radiowave surgery unit for iridocyclectomy and ciliochoroidectomy in rabbits resulted in substantial changes in the structures adjacent to the site of dissection of the iris, ciliary body and choroid. A band of homogenous stroma and dry necrosis of the stroma of the iris and pigment epithelium was observed in the close proximity to the site of dissection (Fig. 4).
There were no technical difficulties in the implementation of surgery with the use of a radiowave surgery unit. In addition, there were sites of dry or wet necrosis of the adjacent choroid and retina. Disorganization of the structure of the sclera was accompanied by an intensive inflammatory infiltration. In some tissue complex specimens (33% of the total number of the obtained tissue complex specimens), homogeneous sclera, uveal tract, retina and remnants of the ciliary body were observed at day 10. In addition, there was evidence of congestion. Marked inflammatory response of all tissues was evidenced by lymphocytic infiltration surrounding the sites of homogenization and necrosis. Peripheral sites were characterized by expanded lumens of blood vessels and diapedetic hemorrhages. At this phase of the study, we observed also a case of wet necrosis of the retina and choroid and disorganized structure of the preserved portion of the ciliary body. Subsequently, there was evidence of cicatrization of the wound canal, with sites of homogeneous sclera and remnants of the iris stroma. In addition, there was evidence of dystrophic changes in large groups of pigment epithelial cells surrounded by mature fibrous tissue. Inflammatory infiltration and necrotic and degenerative changes were generally still observed. There was no significant difference in the frequency of destructive changes between the groups (?2=1.09; p>0.05) due to a small sample size. Discussion Histomorphological studies of the ocular tissues (sclera, iris, ciliary body and choroid) in experimental rabbits demonstrated that after surgery with the use of a radiowave surgery unit, necrotic and degenerative changes were observed in the ocular tissues adjacent to the wound canal which had been preserved intraoperative, and these changes expanded to the retina. Surgery with the use of a radiowave surgery unit was found to be more sparing for tissues compared to surgery with the use of a cutting tool. Thus, marked inflammatory response was noted at day 10 after the use of a former approach, whereas pathomorphotic changes were minimal after iridocyclectomy with the use of the latter approach. At month 1, similar changes were partially maintained, but cicatrization of the wound canal and saved tissues (first of all, subconjunctival, uveal tract and scleral tissues) were more apparent than these changes. We believe that marked pathomorphotic changes developing in the ocular tissues after the use of a radiowave surgery unit is not in disagreement with the prospects of this approach in organ-saving interventions on uveal components, since we used a sparing bandwidth of 3.8-4.0 MHz with a surgery unit set on a monopolar high-frequency current mode, thus reducing the coagulating effect on the tissues. A Surgitron unit can be set on a bipolar mode with a lower frequency, but this is likely to cause even greater tissue alterations than we observed in the current study. Either surgical laser or CyberKnife [3] can be an alternative to a radiowave surgery unit, but the use of these approaches in our country is substantially limited due to economic reasons. Conclusion First, our animal study found that inflammatory response (conjunctival chemosis, conjunctival discharge, and presence of anterior chamber and/or vitreous hemorrhage) was more marked after the use of a cutting tool than after the use of a radiowave surgery unit. Second, after surgery with the use of a radiowave surgery unit, necrotic and degenerative changes were observed in the ocular tissues adjacent to the wound canal which had been preserved intraoperative. Finally, the above changes were maintained, tending to become more severe over 30 days. References 1.Il’in EA. [Bioethics in human and animal studies]. Aviakosmicheskaia I ekologicheskaia meditsina. 2016;50(5):69-77. Russian. 2.Chua V, Aplin AE. Novel therapeutic strategies and targets in advanced uveal melanoma. Curr Opin Oncol. 2018 Mar;30(2):134-141. 3.Kozina EV, Kozina IuV, Gololobov VT, Kokh IA. [Uveal melanoma: major epidemiological aspects and risk factors]. Sibirskoie meditsinskoie obozreniie. 2014; 4(88): 57-64. Russian. 4.Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. 2018 Feb 21;10:1758834018757175. 5.Smoliakova GP, Pikhovskaia IG, Luz’ianina IG, Sorokin EL. [Method for radiosurgical treatment of uveal melanoblastoma]. Russian. Available at http://www.findpatent.ru/patent/226/2265423.html 6.Korobov EN, Iarovoi AA, Gorshkov IM. [Primary endoresection of choroidal melanoma]. Sovremennye tekhnologii v oftalmologii. 2017;1(14):142-5. Russian. 7.Borovikov VP. [Statistica: the art of computerized data analysis]. St Petersburg: Piter;2003. Russian. 8.Gigliotti CR, Modorati G, Di Nicola M, Fiorino C, et al. Predictors of radio-induced visual impairment after radiosurgery for uveal melanoma. Br J Ophthalmol. 2018 Jun;102(6):833-839. 9.Jang BS, Chang JH, Oh S, Lim YJ, Kim IH. Surgery vs radiotherapy in patients with uveal melanoma: Analysis of the SEER database using propensity score matching and weighting. Strahlenther Onkol. 2017 Nov;193(11):931-942. doi: 10.1007/s00066-017-1203-0. 10.Vazhenin AV, Panov IE, Semionova LE, et al. [Our first experience of CyberKnife treatment for choroidal melanoma]. Sibirskii onkologicheskii zhurnal. 2012; (1): 48-50. Russian. 11.Kim JH, Shin SJ, Heo SJ, et al. Prognoses and Clinical Outcomes of Primary and Recurrent Uveal Melanoma. Cancer Res Treat. 2018 Oct; 50(4): 1238–51. 12.Maletskiy AP, Homyakova OV. [Radiowave surgery and seleсtive еndarterial chemotherapy in treatment of patients with uveal melanoma]. In: Proceedings of the Joint Congress SOE/AAO. Geneva, 4-7 June 2011. p.139. 13.Pham C M, Custer PL, Couch SM. Comparison of primary and secondary enucleation for uveal melanoma. Orbit. 2017 Dec;36(6):422-427. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|