J.ophthalmol.(Ukraine).2019;5:56-63.
|
http://doi.org/10.31288/oftalmolzh201955663 Received: 18 April 2019; Published on-line: 30 October 2019 On the role of lipid metabolism and lipid peroxidation in the development of retinal disorders in type 2 diabetic rats with myopia Abdulhadi Mohammad, Cand. Sc. (Med.); I.N. Mikheitseva, Dr. Sc. (Biol.); S.G. Kolomiichuk, a Research Fellow SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Abdulhadi Mohammad, Mikheitseva IN, Kolomiichuk SG. On the role of lipid metabolism and lipid peroxidation in the development of retinal disorders in type 2 diabetic rats with myopia. J.ophthalmol.(Ukraine).2019;5:56-63-48. http://doi.org/10.31288/oftalmolzh201955663
Background. There are current data on the role of dyslipidemia and enhanced processes of lipid peroxidation (LPO) in the pathogenesis of diabetic retinopathy (DR). However, pathogenic mechanisms which can explain the link between main parameters of lipid metabolism and development of DR, especially in high myopia, are still understudied. Purpose. To study the parameters of lipid metabolism and lipid peroxidation in blood and retina of streptozotocin-induced diabetic rats with deprivation myopia with a purpose to reveal the pathogenetic features of the development of type 2 diabetes mellitus in the presence of myopia. Material and Methods. The study was performed on Wistar rats. The rabbits were divided into four groups: group 1, 15 rabbits with axial myopia; group 2, 15 rats with diabetes; group 3, 15 rats with myopia and diabetes; 10 intact rats serving as controls. Eyelids of two-week animals (30 rats) were sutured to induce axial myopia, according to Beuerman R.W. et al. The animals were kept under poor light conditions for 14 days. After a fortnight, the sutures were removed. In two weeks, 15 rats with myopia and 15 intact rats were induced type 2 diabetes mellitus (T2DM). T2DM was induced using 5 daily intraperitoneal injections of streptozotocin (15.0 mg per 1 kg). The control rats were kept under natural light condition. The criterion of diabetes onset was an increase in the blood glucose level up to 4.5mmol/L. After two months, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. To assess myopia severity, axial length was measured post mortem using a digital sliding caliper (Topex) with 0.02 mm accuracy. Levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) [16, 17], diene conjugates (DC), and malondialdehyde (MDA) were evaluated in the blood plasma and DC and MDA levels were measured in the retina. The Atherogenic Index (AI) was calculated. Data obtained were processed statistically with the parametric Student-t test using a software program (Statistica). Results. Expressed disorders were revealed in lipid metabolism, including increased levels of TC, LDL-C, TG, decreased HDL-C, and those in ratios of metabolic parameters in the blood of STZ diabetic rats with and without myopia. No significant changes in the levels of TC, TG, LDL-C, and HDL-C were noted in the rats with deprivation myopia, which gives evidence that there are no disorders in lipid metabolism in the presence of myopia. Our study revealed no significant difference in lipid profile outcomes between the diabetic-only animals and diabetic animals with myopia. Studying LPO parameters in deprivation myopia showed a statistically insignificant increase in the levels of MDA and DC both in the blood plasma and retina of the myopic rats as compared with controls. STZ-induced diabetes resulted in significant changes in the level of LPO products in the rats’ blood plasma and retina. The MDA level was 3.8 times increased in diabetes (р<0.001) and 4.6 times increased in diabetes with myopia (р<0.001) as compared with control. Similar changes were noted in the DC level, which was increased, as compared with control, by 118.8% (р<0.001) and 169.4% (р<0.001) in diabetes only and diabetes with myopia, respectively. Statistically insignificant changes in the levels of LPO were noted in the diabetic rats as compared with the diabetic rats with myopia. Conclusions. Disorders in lipid metabolism parameters in the peripheral blood were revealed both in SZT-induced diabetes and in SZT-induced diabetes in combination with axial myopia. There was no significant difference in lipid metabolism markers between groups with diabetes only and diabetes with axial myopia. Thus, the presence of axial myopia does not worsen lipid metabolism in the SZT-induced diabetic rats. The experiment confirmed the fact that lipid peroxidation is activated in the blood and retina of the SZT-induced diabetic rats; it is also activated in diabetes developed against axial myopia. No significant difference was revealed in the LPO parameters between diabetic rats with and without myopia. Keywords: deprivation myopia, type 2 diabetes mellitus, retina, parameters of lipid metabolism, lipid peroxidation, rats, experiment
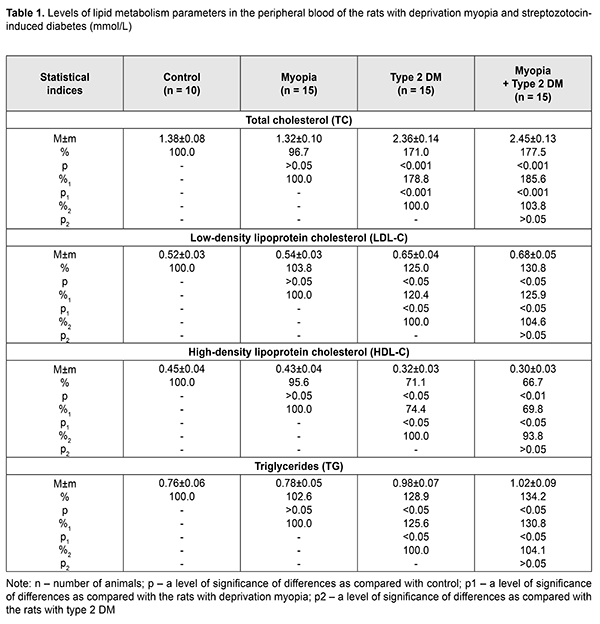
Background Diabetic Mellitus (DM) and diabetic complications are the major causes of disability in the working-age population. In this regard, the problem of studying the pathogenesis of DM as well as prophylaxis and treatment of diabetic retinopathy (DR) is an important social task of ophthalmology [1, 2]. DM of type 1 and 2 are known to be accompanied by processes of lipid peroxidation (LPO) and atherogenic disorders of lipid metabolism [3, 4]. At the same time, dyslipidemia and intense LPO processes have been found to play a significant role in the pathogenesis of DR. Thus, Aldebasi Y.H. and colleagues have reported on increased levels of plasma malondialdehyde, serum cholesterol, triglyceride and low-density lipoprotein and decreased levels of serum high-density lipoprotein in type 2 diabetic patients with proliferative retinopathy. Triglyceride, total cholesterol and lipoprotein have also been found risk factors for developing proliferative DR [5]. Otherwise, Zhou Y. et al. have studied the relationship between dyslipidemia and DR and found no obvious differences in triglycerides, total cholesterol, and high-density lipoprotein cholesterol levels between patients with DR and without DR. However, the paper has reported on the increased low-density lipoprotein cholesterol level in DR [6]. In addition, according to Klein B. E. et al. and Chang, Y. C. (2013), not only low-density lipoprotein cholesterol but also total cholesterol is related with the presence of hard exudates in patients with DR [2, 7]. Today, pathogenetic mechanisms which can explain the link between main parameters of lipid metabolism and development of DR are not completely understood. Based on the literature data, the incidence of non-proliferative and proliferative DR and DR progression is reduced in myopia and, especially, in high myopia [8-11]. A meta-analysis of populational cross-sectional studies has demonstrated that myopia reduces the risk of development of diabetic complications in the retina as compared to emmetropic eyes. Despite the available clinical data indicating that myopic individuals with a longer axial length of globe exhibit a decreased risk of developing DR, a protective mechanism of myopia is unclear. It is likely that in high myopia, vascular and metabolic factors can play a role in a decrease of DR severity. The purpose of the present paper was to study the parameters of lipid metabolism and lipid peroxidation in blood and retina of streptozotocin-induced diabetic rats with deprivation myopia with a purpose to reveal the pathogenetic features of the development of type 2 DM in the presence of myopia. Material and Methods The study was performed on rats following the General Ethical Principles of Animal Experiments (Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986). The rats were divided into four groups: group 1, 15 rabbits with axial myopia; group 2, 15 rats with diabetes; group 3, 15 rats with myopia and diabetes; 10 intact rats serving as controls. Eyelids of two-week animals (30 rats) were sutured to induce axial myopia, according to Beuerman R.W. et al. [12]. The animals were kept under poor light conditions for 14 days, which contributed to achieving a higher rate of myopia [13]. After a fortnight, the sutures were removed. In two weeks, 15 rats with myopia and 15 intact rats were induced type 2 diabetes mellitus (T2DM). T2DM was induced using 5 daily intraperitoneal injections of streptozotocin (15.0 mg per 1 kg). Besides, the rats were fed with hyperlipidemic diet. This would suggest that the animals were induced T2DM. Since, according to Kovaleva et al., the proliferative capacity of the pancreas is decreased with age, streptozotocin-induce diabetes in 4-5-week rats can develop T2DM [14]. Based on the data of Kolbin et al., streptozotocin diabetes which was induced in newborn rats fed with hyperlipidemic diet developed T2DM [15]. The control rats were kept under natural light condition. Throughout the experiment, a blood glucose level was measured using a ME-DC (Germany) glucometer. The criterion of diabetes onset was an increase in the blood glucose level up to 4.5mmol/L and higher with 6-8 week age norm defined as (2.80±0.13) mmol/L. After two months, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. To assess myopia severity, axial length was measured post mortem using a digital sliding caliper (Topex) with 0.02 mm accuracy. Data obtained were processed using the non-parametric tests. Levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) [16, 17], diene conjugates (DC), and malondialdehyde (MDA) were evaluated in the blood plasma and DC and MDA levels were measured in the retina. The Atherogenic Index (AI) was calculated using the following formula: AI = (TC - HDL-C) / HDL-C and ratios: HDL-C/ LDL-C, TG/HDL-C. Data obtained were processed statistically with the parametric Student-t criterion using a software program (Statistica). Results and Discussion Following 5 intraperitoneal injections of streptozotocin (STZ), the glucose level ranged between 4.5 and 7.9 mmol/l, equaling (5.11±0.29) mmol/l. The glucose level was significantly increased in the STZ diabetic rats, by 82.5% as compared to the intact rats. We evaluated the state of lipid metabolism in all four groups by measuring TC, TG, HDL-C and LDL-C levels (Table 1). In the peripheral blood, TC was significantly increased in the STZ diabetic rats and STZ diabetic rats with myopia, by 71.0% (р<0.001) and 77.5% (р<0.001), respectively, compared with controls. In the rats with deprivation myopia only, the glucose level was within the control values. In the blood plasma of the STZ diabetic rats, the LDL-C level was increased by 25.0% while the HDL-C level was decreased by 28.9% as compared with control (р<0.05). In the diabetic rats with myopia, the LDL-C level was increased by 30.8% (р<0.05) while the HDL-C level was decreased by 33.3% (р<0.01) as compared with control. These parameters of lipid metabolism did not change significantly in the group of rats with myopia only. An increased TG level was revealed in the blood plasma of the diabetic rats and diabetic rats with myopia, by 34.2% (р<0.05) and 28.9% (р<0.05), respectively, as compared to control (Table 1). No changes in the TG level were noted in the myopic rats.
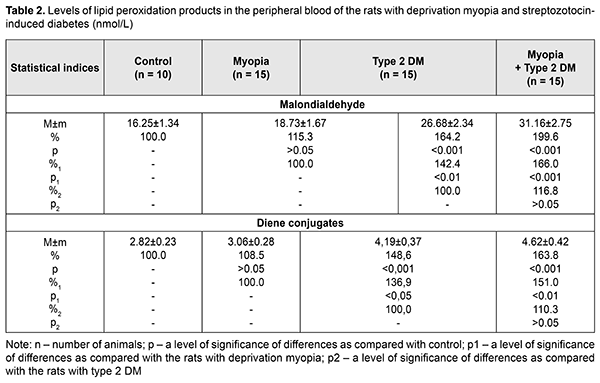
Besides, the Atherogenic Index was assessed for the blood of the studied animals. AI was equal to 6.38 and 7.17 in diabetes-only and diabetes with myopia, respectively, vs. 2.07 in control. Herewith, an HDL-C/LDL-C ratio was characterized by lower values in the diabetic rats both without and with myopia, 0.49 and 0.44, respectively, while that was 0.80 and 0.87 in the myopic rats and control, respectively. Based on epidemiological studies, changes in the pointed ratios are an important risk factor for cardiovascular system disorders [19]. Assessing a TG/HDL-C ratio, which can be used as an insulin resistance marker [20], it was found increased in the blood plasma of the diabetic rats due to increased TG and decreased HDL-C: by 3.06 and 3.4 in the diabetic rats and diabetic rats with myopia, respectively, vs. 1.69 in control. Thus, assessing lipid profile outcomes of the study groups, it can be concluded that pronounced STZ diabetes-induced disorders in lipid metabolism were noted in groups 2 and 3. No significant changes in TC, TG, LDL-C, and HDL-C levels were revealed in the animals with deprivation myopia, which gives evidence of the absence of disorders in lipid metabolism in myopia development. Our study revealed no significant difference in lipid profile outcomes between the animals with diabetes only and animals with diabetes and myopia. Consequently, myopia did not worsen the disorders in lipid metabolism in the rats with SZT diabetes. According to different authors, as a rule, lipid metabolism is disordered in diabetes mellitus with increased levels of cholesterol, triglycerides, and other compounds. In turn, dyslipidemia, over time, increases significantly the risk of ischemia in tissues and atherosclerosis [21–24]. The following stage of the work was studying the LPO processes and determining such markers as MDA and DC in the blood and retina of the studied rats. Biochemical studies of LPO parameters in deprivation myopia showed a tendency to an increase in the intensity of the LPO process in the blood and retina (Tables 2. 3). A statistically insignificant increase was noted in MDA and DC levels both in the blood and retina of the myopic rats as compared with control.
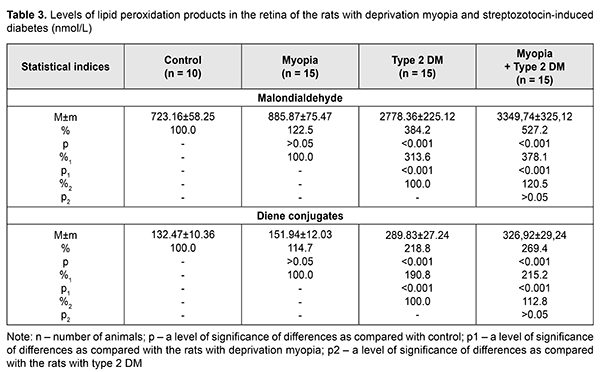
SZT diabetes caused changes in LPO products in the rats’ blood: MDA and DC levels were increased by 64.2% (р<0.001) and 48.6% (р<0.001), respectively, as compared with control and by 42.4% (р<0.01) and 36.9% (р<0.05), respectively, as compared with the rats with myopia (Table 2). The MDA and DC levels were even more increased in the diabetic rats with myopia, equaling 199.6% (р<0.001) and 163.8% (р<0.001), respectively, as compared with control and 166.0% (р<0.001) and 151.0% (р<0.01), respectively, as compared with the rats with myopia only. Comparing the data obtained from the diabetic rats with myopia, we found that the MDA and DC levels were increased by 16.8% (р>0.05) and 10.3% (р>0.05), respectively, as compared with diabetic rats without myopia. LPO parameters in the rats’ retina were insignificantly increased in myopia and more pronounced changes in both NDA and DC levels were noted in the retina of diabetic rats as compared with control (Table3). Thus, the MDA level in the retina was increased 3.8 times (р<0.001) and 4.6 times (р<0.001) in the rats with diabetes and diabetic rats with myopia, respectively, compared with control. Similar changes were noted in DC levels in the retina of the studied rats, showing an increase by 118.8% (р<0.001) and 169.4% (р<0.001) in the diabetic rats and diabetic rats with myopia, respectively, compared with control. Comparison of the LPO data obtained from the retinas of the studied groups showed that the DC level was increased by 90.8% (р<0.001) and 115.2% (р<0.001) in group 2 with diabetes and group 3 with diabetes and myopia, as compared with group 1 with myopia. The MDA and DC levels in the diabetic rats with myopia were increased by 20.5% and 12.8%, respectively, compared with diabetic rats without myopia. However, the difference between the groups was not statistically significant (р>0.05) so we can speak only about a tendency to their increase. Thus, we obtained the data supporting the fact that LPO processes are activated in experimental animals with diabetes. Moreover, it was found that when diabetes was induced in the rats with a longer axial length due to a model of axial myopia, metabolic changes, especially in the retina tissues, were more pronounced although the difference was not statistically significant. We found that the LPO processes were intense in the blood and retina of the rats with axial myopia, which confirms the data of other scientists. Thus, several papers have reported on the absence of significant changes in LPO product levels but only a tendency to increase. Eun Bi Kim et al. have shown no significant differences in MDA levels in the aqueous humor in patients with high myopia compared to control (aqueous humor after cataract extraction) and no significant correlation with axial length [25]. At the same time, there are papers demonstrating increased levels of LPO products and decreased activity of the antioxidant system in the blood of children with myopia. Thus, however, some papers highlight a certain role of oxidative stress in the development of myopia, especially in the combination of other eye diseases [28, 29], including diabetes [31, 31]. Yuri K. S. and colleagues have noted alterations in lipid profile outcomes in four-month rats with neonatally-induced SZT diabetes as follows: levels of LPO, triglycerides, and cholesterol tended to be increased, however, due to variability, the data obtained did not differ significantly from the control group [32]. Lipid metabolism disorders are alleged to play a certain role in the development of diabetic microangiopathy [33]. Herewith, patients with microvascular complication and increased glycated hemoglobin (> 8%), including those with diabetic retinopathy, had significantly increased levels of total cholesterol and atherogenic lipids [34]. According to Nagra P.K. and colleagues, triglyceridemia can increase significantly the risk of retinal vascular occlusion [35]. One of the possible mechanisms of the pathogenic action of increased lipid metabolism parameters can be considered a possibility of lipoprotein diffusion into the retinal extracellular space with the formation therein of exudates and macular edema [1]. Taking into account that the retina has a high oxidative activity, modification of lipoproteins can contribute to the accumulation of LPO products, an increased level of which has a pathogenetic role for diabetic complications [2]. Also, LPO levels in the retinal vascular tissues correlate with those in the blood serum and are associated with severity of diabetic retinopathy in patients. Increased levels of MDA and other free-radical lipid oxidation products in blood in diabetes enhance peroxidation processes in a lipoprotein complex and, thus, can affect the affinity of lipoproteins to their receptors and cause the formation of cholesteric thickening in the tissue vessels, including those in ocular tissues [36, 37]. Furthermore, accumulation of LPO products in diabetic patients contributes to the stiffening of cell membranes with a decreased unsaturated fatty acid level, which changes their functional activity, decreases an enzyme-coupling capacity of insulin receptors and glucose consumption by cells [4, 38]. It should also be noted that the retina, which is characterized by a strong need in energy metabolism, is under conditions of constant light effects, which causes increased sensitivity to oxidative stress [39]. That’s why oxidative stress today is considered as an important pathogenetic factor in the development of diabetic retinopathy in type 1 and 2 diabetes mellitus [40]. Features of metabolism in the blood and retina of type 2 diabetic animals with axial myopia, which we revealed in the experiment, can be relevant for determining the mechanism of mutual effect between myopia and diabetic complications. Conclusions Firstly, we revealed disorders in lipid metabolism parameters (increased levels of total cholesterols, triglycerides, low-density lipoprotein cholesterol and decreased high-density lipoprotein cholesterol) in the peripheral blood both in SZT-induced diabetes and in SZT-induced diabetes in combination with axial myopia. Herewith, there was no significant difference in lipid metabolism markers between groups with diabetes only and diabetes with axial myopia. Thus, the presence of axial myopia does not worsen lipid metabolism in SZT-induced diabetic rats. Secondly, the experiment confirmed the fact that lipid peroxidation is activated in the blood and retina of the SZT-induced diabetic rats, evidenced by increased levels of diene conjugates and malondialdehyde; it is also activated in diabetes developed against axial myopia. No significant difference was revealed in the LPO parameters between diabetic rats with and without myopia. References 1.[Diabetes Mellitus: acute and chronic complications]. Edited by Dedov I.I., Shestakova M.V. Moscow: Med. Inform. Agenstvo;2012. 480p. In Russian. 2.Chang YC, Wu WC. Dyslipidemia and Diabetic Retinopathy. The Review of Diabetic Studies. 2013;10(2–3):121–32. 3.Mikaeliyan NP, Gurina AE, Nguen NZ et al. [The relationship between process of peroxidation of lipids, activity of antioxidant system and fatty-acid composition of blood in patients with diabetes mellitus type I and under its complications]. Ros. Med. Zhurn. 2014;4:33-8. In Russian. 4.Mikaelyan NP, Potemkin VV. [The metabolic disorders in membrane of erythrocytes and insulin-binding activity of blood cells in patients under obesity and diabetes mellitus type II]. Ros. Med. Zhurn. 2017;23(4):201-4. In Russian. 5.Aldebasi YH, Mohieldein АН, Almansour YS, Almutairi BL. Dyslipidemia and lipid peroxidation of Saudi type 2 diabetics with proliferative retinopathy. Saudi Med. J. 2013;34(6): 616–22. 6.Zhou Yue, Changyun Wang, Ke Shi et al. Relationship between dyslipidemia and diabetic retinopathy A systematic review and meta-analysis. Medicine (Baltimore). 2018; Sep; 97(36): e12283. Published online 2018 Sep 7. 7.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudates. Ophthalmology. 1991;98(8):1261–5. 8.Tayyab H, Haider МА, Bukhari Shaheed, Haider SА. Axial myopia and its influence on diabetic retinopathy. J. Coll. Physicians Surg. Pak. 2014. Oct; 24(10): 728-31. doi: 10.2014/JCPSP.728731. 9.Wang X, Tang L, Gao L, Yang Y, Cao D, Li Y. Myopia and diabetic retinopathy: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016 Jan;111:1-9. doi: 10.1016/j.diabres.2015.10.020. Epub 2015 Oct 23. 10.Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016. 22(6):589-99. 11.Bazzazi N, Akbarzadeh S, Yavarikia М et al. High myopia and diabetic retinopathy: A Contralateral Eye Study in Diabetic Patients With High Myopic Anisometropia. Retina. 2017. Jul; 37(7):1270-6. 12.Beuerman RW, Maw SS, Tan DT et al. Myopia: animal models to clinical trials. Singapore World Scientific; 2010. 390 p. 13.Mikheytseva IN, Abdulhadi Mohammad, Putienko АА et al. Modelling form deprivation myopia in experiment. J.ophthalmol.(Ukraine).2018;2:50-55. 14.Kovaleva MA, Kryshen KL, Makarova MN, Makarov VG. [Age of formation of streptozotocin-induced diabetes in rats]. Mezhdunarodnyi vestnik veterinarii. 2014;4:90-6. In Russian. 15.Kolbina MV, Chesnokov VI, Dolgich VT. [The particularities of model ingof type 2 diabetes mellitusin rats]. Vestnik KazNMU.2013;5(1):145-7. In Russian. 16.Chaialo PP. [Disorders of lipoprotein metabolism]. K.:Zdorovya;1990. 184p. In Russian. 17.Goriachkovskii AM. [Clinical biochemistry in laboratory diagnostics]. Odessa:Ekologiia; 2005. 616p. In Russian. 18.Orekhovich VN. [Modern methods in biochemistry]. Moscow: Meditsina; 1977: 392p. In Russian. 19.Enomoto М, Adachi H, Hirai Y et al. LDL-C/HDL-C Ratio Predicts Carotid Intima-Media Thickness Progression Better Than HDL-C or LDL-C Alone. J. Lipids. 2011. Article ID 549137, 6 pages 20.Haymana С, Sonmez A, Aydoqdu A et al. Visceral adiposity index and triglyceride/high-density lipoprotein cholesterol ratio in hypogonadism. Arch Endocrinol Metab. 2017; 61(3):282 – 7. 21.Cowie CC, Harris MI. Physical and metabolic characteristics of persons with diabetes. Diabetes in America. 2nd ed. Washington (DC): National Institutes of Health, 1995:117–64. 22.Syv?nne M, Taskinen MR. Lipids and lipoproteins as coronary risk factors in non-insulin-dependent Diabetes mellitus. Lancet. 1997; 350:20–3. 23.Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiol. Rev. 2006;14:125–35. 24.Protasov KV. [Atherogenic dyslipidemia in diabetes mellitus. Part 1: pathogenesis, clinical and prognostic significance, lipid level monitoring indices]. Sibirskii Med. zhurn. 2012;5:5-9. In Russian. 25.Eun Bi Kim, Ha Kyoung Kim, Joon Young Hyon et al. Oxidative Stress Levels in Aqueous Humor from High Myopic Patients. Korean J. Ophthalmol. 2016; 30(3):172–9. 26.Boichuk I. M., Surovaya K. I., Kolomijchuk S. G. [Content of the free and connected albuminous sulfide and disulfide groups in tear fluid depending on the degree of myopia in children]. Oftalmol Zh. 2015;1:41-4. In Russian. 27.Khamnagdayeva NV, Obrubov SA, Semenova LY et al. [Regularities of changes in the levels of malondialdehyde and retinol in blood serum of children with myopia in case of poly-morbidity]. Ros. detskaia oftalmologiia.2017;1:35-9. In Russian. 28.Micelli-Ferrari T, Vendemiale G, Grattagliano I et al. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. British Journal of Ophthalmology. 1996;80(9):840–3. 29.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015:4:180–3. 30.Seghrouchi I, Dari J, Bannier Е et al. Oxidative stress parameters in type 1, type 2 and insulin-treated type 2 diabetes mellitus; insulin treatment efficacy. Clin Chim Acta. 2002;321:89–96. 31.Cui Y, Хu Х, Zhu Q et al. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Experimental Eye Research. 2006;83(4):807–16. 32.Yuri KS, Paula HО, Kleber Eduardo de Campos et al. Neonatally-induced diabetes: lipid profile outcomes and oxidative stress status in adult rats. Rev. Assoc. Med. Bras. 2009; 55(4):384–8. 33.Samoilova YuG, Yurchenko YeV. [Characteristics of lipid metabolism in patients with type 1 diabetes, depending on the availability of diabetic microangiopathy and diet]. Bulleten sibirskoi meditsiny. 2014;5:87-92. In Russian. 34.Syomshchikov VS, Khamnueva LYu, Chugunova EV. [Abnormality of lipid metabolism in patients with diabetes mellitus type 1 and poor glycemic control with and without diabetic microangiopathies]. Acta Biomedica Scientifica. 2016;1(6):113-17. In Russian. 35.Nagra PK, Ho АС, Dugan JDJr. Lipemia retinalis associated with branch retinal vein occlusion. Am. J. Ophthalmol. 2003;135:539–42. 36.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865): 813–20. 37.Novitsky VV, Kravets YeВ, Kolosova MV et al. [The lipid spectrum of erythrocyte membranes in children with diabetes mellitus]. Problemy endokrinologii. 2006;52(4):3-6. In Russian. 38.Potemkin VV, Frantseva EYu., Kulaieva IO, Mikaelyan NP. [Functional state of membrane-receptor system of the blood in newly diagnosed type 2 diabetes mellitus]. Problemy endokrinologii. 2012;4(2):40-1. In Russian. 39.Ceriello А, Esposito К, Ihnat М et al. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J. Clin. Endocrinol. Metab. 2009;94(8):2751–6. 40.Pannicke Т, Iandiev I, Wurm А et al. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55(3):633–9.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|