J.ophthalmol.(Ukraine).2020;1:35-39.
|
http://doi.org/10.31288/oftalmolzh202013539 Received: 17 December 2019; Published on-line: 21 February 2020 Early signs of primary compressive optic atrophy evidenced by OCT in patients with basal brain tumors K.S. Iegorova,1 Cand Sc (Med); M.A. Znamenska,2 Dr Sc (Med); M.O. Guk,1 Dr Sc (Med); A.O. Mumliev,1 Cand Sc (Med) 1 Romodanov Neurosurgery Institute, National Academy of Medical Science of Ukraine; Kyiv (Ukraine) 2 Institute of Pediatrics, Obstetrics and Gynecology, National Academy of Medical Science of Ukraine; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE: Iegorova KS, Znamenska MA, Guk MO, Mumliev AO. Early signs of primary compressive optic atrophy evidenced by OCT in patients with basal brain tumors. J.ophthalmol.(Ukraine).2020;1:35-39. http://doi.org/10.31288/oftalmolzh202013539
Background: Compression of the optic nerve/chiasm complex is typical for patients with basal neoplasms located in the middle or anterior cranial fossae. The most common of these neoplasms are pituitary adenomas, meningiomas and craniopharyngiomas. Chiasmal compression is accompanied by a gradual decrease in visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA). Optical coherence tomography (OCT) is a relatively new non-invasive imaging modality that allows objective assessment of stereometric parameters of the optic nerve. Purpose: To assess OCT-measured morphostructural parameters of the retina and optic nerve in patients with primary optic atrophy due to compression by basal brain tumors (BBT). Material and Methods: This retrospective study included the records of 43 patients (86 eyes) who received treatment for BBT at the Romodanov Institute during 2016 to 2017. Patients underwent clinical and neurological, eye, and otoneurological examination, and neuroimaging procedures, particularly OCT with a Revo NX instrument (Optopol Technology SA, Poland). Results: The OCT features of compressive descending OA varied with the severity of chiasmal syndrome (CS). The peripapillary retinal nerve fiber layer (RNFL) thickness was statistically significantly different between patients with CS versus controls and versus patients with no CS. In addition, there was a significant difference (p < 0.05) in reduction in neuroretinal rim area in patients with CS, compared with controls. The macular ganglion cell complex (GCC) thickness was thinner in each subgroup of patients than in the control group. There were significant direct correlations of visual field mean deviation (MD) with RNFL thickness and GCC thickness. Conclusion: OCT is a modern non-invasive objective modality that can be utilized in the diagnosis of primary OA, and allows quantifying the reduction in nerve fiber thickness. The GCC thickness analysis provides earlier diagnosis of chiasmal compression than identification of temporal visual field defects or analysis of peripapillar RNFL thickness. Keywords: basal brain tumors, chiasmal syndrome, optic atrophy, optical coherence tomography
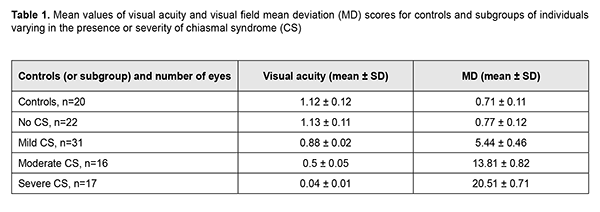
Introduction Compression of the optic nerve/chiasm complex is typical for patients with basal neoplasms located in the middle or anterior cranial fossae. The most common of these neoplasms are pituitary adenomas, meningiomas and craniopharyngiomas [1-3]. Chiasmal compression is accompanied by a gradual decrease in visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA). Clinical manifestations of basal brain tumors (BBTs) vary and depend on tumor involvement, nature of growth pattern, and rate of growth. Impaired visual functions are observed in 40-65% of patients, and commonly are the first manifestation of the disease [4, 5]. Late-diagnosed but mostly benign processes causing compression of the optic nerve/chiasm complex may result in temporary or permanent visual function loss leading to visual disability. In spite of advances in early imaging diagnosis (in particular, subclinical diagnosis) of BBT, the annual number of newly registered cases of compressive optic atrophy associated with BBT is not decreasing, according to the Neurosurgery Institute. Ophthalmoscopy is the most common objective method of diagnosing OA in Ukraine; it is, however, somewhat subjective since it does not allow quantification of the degree of OA, and characteristics of the optic disc structure vary from individual to individual. Optical coherence tomography (OCT) is a relatively new non-invasive imaging modality that allows objective assessment of stereometric parameters of the optic nerve [6, 7]. A role for the use of OCT in identifying RNFL thickness loss in eyes with OA has been suggested [8]. The degree of OCT-measured RNFL thickness reduction has been shown to correlate with that of visual field defects as assessed by static automated perimetry [9] and Goldmann perimetry [10]. It has been found [8, 11] that the overall OCT-measured RNFL thickness did not correlate well with neural capacity, and concluded that sensitivity of RNFL thickness measurement in OCT for eyes with compression of the optic chiasm by a BTT was low, and the method has limited value in the diagnosis of pituitary tumour compression. The purpose of this study was to assess OCT-measured morphostructural parameters of the retina and optic nerve in patients with primary optic atrophy due to compression by BBT. Material and Methods This retrospective study included the records of 43 patients (86 eyes; 27 women (63%) and 16 men (37); age, 28 to 77 years; mean age, 50 ± 1.6 years) who received treatment for BBT at the Romodanov Institute during 2016 to 2017. The inclusion criterion was neutoimaging evidence of BBT causing chiasmal compression. Exclusion criteria were patients with continued tumor growth, signs of intracranial hypertension or concomitant eye disorders. Patients underwent clinical and neurological, ophthalmic, and otoneurological examination. Instrumental and laboratory studies were conducted. Neuroimaging procedures (sella turcica X-ray study, magnetic resonance imaging (MRT), and computer tomography (CT)) were performed. An ophthalmic examination was conducted 12 months after tumor removal (chiasmal and optic nerve decompression) and included best-corrected visual acuity (BCVA) assessment, biomicroscopy, kinetic and static perimetry, direct and indirect ophthalmoscopy, and OCT. Static automated perimetry (SAP) was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) using the neurological 30-2 threshold test program and Neuro screening program. OCT was conducted using a Revo NX instrument (Optopol Technology SA, Zawiercie, Poland). Optic disc excavation area, depth and volume, peripapillary RNFL thickness, reduction in neuroretinal rim (NRR) area, and macular ganglion cell complex (GCC) thickness were assessed by OCT. The main group included 43 patients (86 eyes) with BTT. These were divided into subgroups taking into account the severity of chiasmal syndrome (CS) (BCVA and visual fields as assessed by mean deviation (MD)): Subgroup 1 of 22 eyes with no CS (BCVA, 1.0 or better; MD, -2 dB or better) in the presence of compression of the optic nerve/chiasm complex by the neoplasm based on neuroimaging (MRI or CT) evidence; Subgroup 2 of 31 eyes with mild CS (BCVA, 0.5 to 0.9; MD, -2 to -10 dB); Subgroup 3 of 16 eyes with moderate CS (BCVA, 0.1 to 0.4; MD, -10 to -20 dB); and Subgroup 4 of 17 eyes with severe CS (BCVA, <0.1 to 0.4 and/or MD worse than -20 dB). The control group comprised 10 individuals (20 eyes) with no concomitant ocular or neurosurgical disorder. This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. The data were statistically processed using Statistica 6.0 (StatSoft, Tulsa, OK). Results are presented as the mean and standard deviation (M ± SD). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ? 0.05 was assumed. Spearman correlation coefficients were used to assess correlations. Results and Discussion Pituitary adenoma (PA) was the most common BBT (29 cases; 67.4 % of patients), followed by tuberculum sellae meningioma (9 cases; 20.9 %), craniopharyngioma (2 cases; 4.6 %), and germinoma (1 case; 2.3 %). Tumor extension was classified as suprasellar in all cases, and visual impairment was the major manifestation of the disease in most patients. Of the 43 patients, 41 underwent chiasmal and optic nerve decompression by tumor removal, and 2 had involution of tumor (prolactinoma) after conservative treatment. The major sign of BBT is a gradual development of chiasmal syndrome, which is accompanied by decreased visual acuity, bitemporal visual field defects and development of primary descending OA. There were significant differences in visual acuity and MD between patients with mild, moderate and severe chasmal syndrome and controls (p < 0.05), but not between patients with no chiasmal syndrome and controls (p > 0.05). Table 1 presents mean visual acuity and MD values for subgroups of patients and controls.
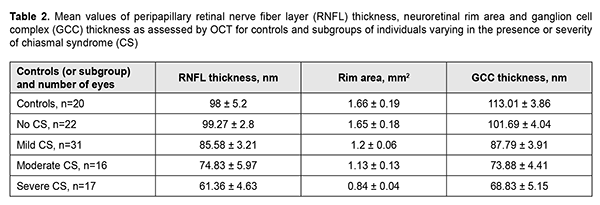
Among the subgroup of patients with mild CS, BCVA varied from 0.6 to 0.1 (mean value, 0.88 ± 0.02), and 14 eyes had relative temporal hemianopia; 1 eye, absolute temporal hemianopia; 8 eyes, relative superior quadrant hemianopia; 3 eyes, temporal visual field constriction; and 1 eye, paracentral temporal scotoma. Among the subgroup of patients with moderate CS, BCVA varied from 0.2 to 0.9 (mean value, 0.5 ± 0.05), and 8 eyes had absolute temporal hemianopia; 3 eyes, relative temporal hemianopia; 3 eyes, temporal hemianopia with central scotoma; 1 eye, central scotoma only; and 1 eye, residual visual field in the nasal hemisphere. Among the subgroup of patients with severe CS, visual acuity varied from amaurosis to 0.1 (mean value, 0.04 ± 0.01), and 9 eyes had residual visual field; and 4 eyes, absolute temporal hemianopia with central scotoma. In addition, visual field was not measurable in another 4 eyes of this subgroup. The OCT features of compressive descending OA varied with the severity of CS (Table 2). There was a significant difference (p < 0.05) in mean RNFL thickness in patients who had mild CS (92.58 ± 3.21 nm), moderate CS (74.83 ± 5.97 nm), and severe CS (61.36 ± 4.63 nm), compared with controls (98 ± 5.2 nm) and patients who had no CS (99.27 ± 4.8 nm). The RNFL thickness loss was noted mostly in the nasal quadrant for those with mild or moderate CS, and in the superior, nasal and inferior quadrants for those with severe CS. In all 17 eyes with severe CS, 11 eyes (68.8%) with moderate CS, and 7 eyes (22.6%) with mild CS, there was ophthalmoscopic evidence of primary descending OA. Retinal angiopathy was observed in the fundus of eyes with no CS.
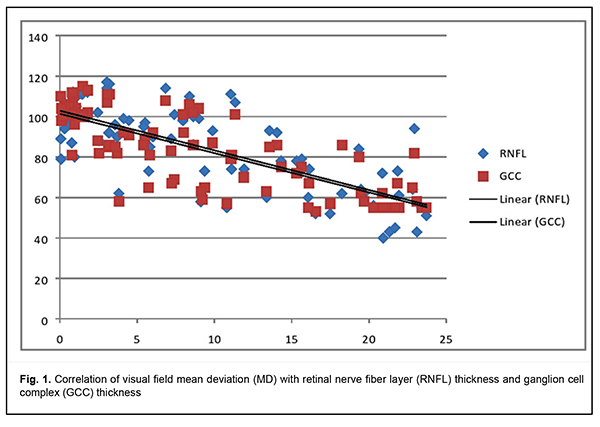
In addition, there was a significant difference (p < 0.05) in reduction in NRR area in patients who had mild CS (1.5 ± 0.06 mm2), moderate CS (1.13 ± 0.13 mm2), and severe CS (0.84 ± 0.04 mm2), compared with controls (1.66 ± 0.19 mm2) and patients who had no CS (1.65 ± 0.18 mm2). Moreover, there was a significant difference (p < 0.05) in GCC thickness in patients who had no CS (110.01 ± 4.04 nm), patients who had mild CS (87.79 ± 3.91 nm), moderate CS (73.88 ± 4.41 nm), and severe CS (68.83 ± 5.15 nm), compared with controls (113.01 ± 3.86 nm). The GCC thickness loss was noted in the nasal quadrant for those with no or mild CS, and in all quadrants for those with moderate or severe CS. Mean deviation (MD) correlated with RNFL thickness and GCC thickness (Fig. 1).
There was a significant direct correlation between RNFL thickness and GCC thickness. Correlation of GCC thickness with MD (r2=0.6009) was stronger compared to that of RNFL thickness (r2=0.5431). There was a significant difference (p < 0.05) in mean RNFL thickness in patients who had CS, compared with controls and patients who had no CS. Reduction in NRR area was significantly less in patients who had CS, compared with controls. BBT is a common cause of chiasmal compression, leading to chiasmal syndrome and primary descending OA. In patients with long-standing compression of the optic chiasm, ganglion cells may undergo axonal degeneration, with axonal loss in the optic nerve and retina manifesting as reduced peripapillary RNFL thickness, reduced NRR area and reduced GCC thickness. In the MRI evident chiasmal compression and in the absence of visual impairment, patients exhibited mean GCC thickness as low as 110.01 ± 4.04 nm. Binasal loss of retinal ganglion cells precedes bitermporal (heteronymous) hemianopsia, reduced visual acuity, reduced RNFL thickness, and reduced NRR area, and is an early diagnostic sign of nerve fiber atrophy and visual function loss in basal brain tumors. Reduced peripapillary RNFL thickness, reduced NRR area, and reduced GCC thickness were observed in the presence of chiasmal syndrome. MD correlated with GCC thickness (r2=0.6009) and with RNFL thickness (r2=0.5431). Conclusion OCT is a modern non-invasive objective modality that can be utilized in the subclinical diagnosis of primary optic atrophy due to compression by BBT, and allows quantifying the reduction in nerve fiber thickness. The GCC thickness analysis provides earlier diagnosis of chiasmal compression than identification of temporal visual field defects or analysis of peripapillar RNFL thickness.
References 1.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Annals of Endocrinology. 2015 Jul;76(3):210-9. doi: 10.1016/j.ando.2015.04.006. 2.Predoi D, Badiu C, Alexandrescu D, Agarbiceanu C, Stangu C, Ogrezeanu I, et al. Assessment of compressive optic neuropathy in long standing pituitary macroadenomas. Acta Endocrinologica (Buc). 2008 Jan; 4(1):11-22. 3.Guk MO. [Diagnosis and comprehensive treatment of hormonally-inactive pituitary adenomas]. Thesis for Doctor of Medical Sciences. Kyiv. 2017. Ukrainian. 4.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 5.Serova NK. [Clinical neuroophthalmology. Neurosurgery aspects]. Tver’: Triada; 2011. Russian. 6.Frohman E, Fujimoto J, Frohman T, Calabresi P, Cutter G, Balcer L. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008 Dec;4(12):664-75. doi: 10.1038/ncpneuro0950. 7.Schuman J, Hee M, Arya A, Pedut-Kloizman T, Puliafito C.A, Fujimoto J.F, Swanson EA. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995; 6:89–95. 8.Johansson C, Lindblom B. The role of optical coherence tomography in the detection of pituitary adenoma. 2009 Nov;87(7):776-9. doi: 10.1111/j.1755-3768.2008.01344.x. 9.Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y, Vander Hoorn S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4827-35. 10.Kanamori A, Nakamura M, Matsui N, et al. Optical coherence tomography detects characteristic retinal nerve fiber layer thickness corresponding to band atrophy of the optic disc. Ophthalmology. 2004 Dec;111(12):2278-83. 11.Tieger MG, Hedges TR 3rd, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, Mendoza-Santiesteban CE. Ganglion Cell Complex Loss in Chiasmal Compression by Brain Tumors. J Neuroophthalmol. 2017 Mar;37(1):7-12. doi: 10.1097/WNO.0000000000000424.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|