J.ophthalmol.(Ukraine).2020;2:56-59.
|
http://doi.org/10.31288/oftalmolzh202025659 Received: 02 April 2020; Published on-line: 30 April 2020 Late immunocorrection after frontal orbital trauma surgery that involved the use of biocomposite О.D. Bondarchuk1, 2, Cand Sc (Med); V.V. Kishchuk 1, Dr Sc (Med), Prof.; О.F. Melnykov 3, Dr Sc (Med), Prof.; М.D. Tymchenko 3, Cand Sc (Biol); N.D. Didyk 2, MD 1 Pirogov National Medical University of Vinnytsia; Vinnytsia (Ukraine) 2 Pirogov Regional Clinical Hospital of Vinnytsia; Vinnytsia (Ukraine) 3 Prof. Kolomiichenko Institute of Otolaryngology, NAMS of Ukraine; Kyiv (Ukraine) E-mail: eskulap20009@gmail.com TO CITE THIS ARTICLE: Bondarchuk ОD, Kishchuk VV, Melnykov ОF, Tymchenko МD, Didyk ND. Late immunocorrection after frontal rbital trauma surgery that involved the use of biocomposite. J.ophthalmol.(Ukraine).2020;2:56-59. http://doi.org/10.31288/oftalmolzh202025659
Purpose: To correct immune system deficiencies in patients with a problematic recovery course after frontal orbital trauma surgery that involved the use of SYNTEKIST biocomposite. Material and Methods: Twenty facial bone trauma (FBT) patients with complicated wound healing in the late postoperative period were examined. The control group involved 10 healthy individuals. Patients of the rehabilitation treatment group were administered Imupret®, the commercial herbal medicinal product and an immunocorrective and antiphlogistic agent, and Eebisol, an IFN-? inducer and repairing agent. Serum levels of immunoglobulin (Ig)M, IgE, interleukin (IL)-1?, IL-10, gamma interferon (IFN-?), and transforming growth factor (TGF)-1? were measured. Results: Two groups of patients who underwent FBT surgery that involved the use of biocomposite were identified. The group with a problematic late postoperative course exhibited stimulation of pro- and anti-inflammatory cytokines and absence of changes in serum levels of immunoglobulins and circulating immune complexes compared to controls and the group with uneventful late postoperative course. In addition, the group with a problematic late postoperative course exhibited decreased serum levels of TGF-? and IFN-?, which may be considered as pathogenetically significant factors involved in regeneration processes. No humoral autoimmune response to connective tissue antigens was found. Conclusion: Increased serum levels of pro- and anti-inflammatory interleukins and decreased serum levels of TGF-? and, especially, IFN-?, are observed in patients with problematic regeneration. No humoral autoimmune response to connective tissue antigens was found in either group of patients with FBT. Stress mechanisms might be involved in decreased immunity levels in these patients. Keywords: frontal orbital trauma, immunoglobulins, cytokines, growth factor, immune complexes, humoral autoimmune responses, SYNTEKIST biocomposite
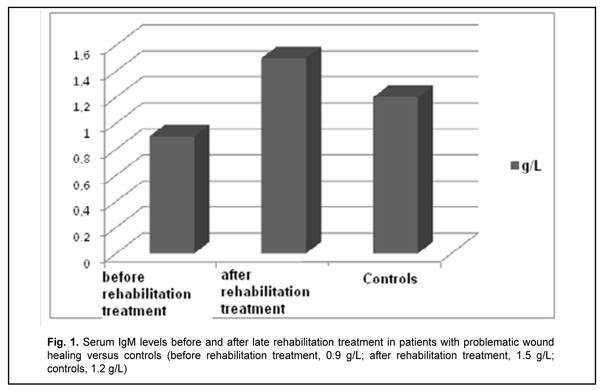
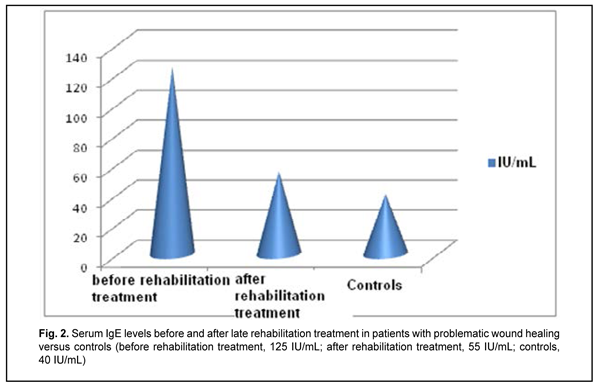
Introduction Hydroxyapatite-based biocomposites (such as SYNTEKIST) have been used in surgical treatment of frontal bone trauma (FBT) in recent years, and are capable of facilitating ostheogenesis and preventing liquorrhea and other post-traumatic sequelae (Kishchuk, Bondarchuk, 2007; Kishchuk, Bondarchuk, et al, 2018). Although most patients exhibit an uneventful course, the rest exhibit a problematic course involving untimely healing of wound, liquorrhea and systemic immunity factors relevant to regeneration after the surgical treatment of FBT. For example, the latter patients had decreased transforming growth factor (TGF)-1? and IFN-? levels, and increased proinflammatory cytokine interleukin (IL)-1? and immunoglobulin (Ig)E levels. Given the revealed abnormalities in patients with a problematic course, we proposed the rehabilitation treatment regimen involving an immunocorrective agent, IFN-? inducer, antihistamine agent and vitamins A, D and E. Material and Methods Ten patients underwent rehabilitation treatment for problematic wound healing after facial bone trauma surgery that involved the use of SYNTEKIST biocomposite. Given the altered systemic immunity, and in line with recommendations of the leading clinical immunologists (Drannik, 2010; Khaitov, Ataullakhanov, 2012) on immune rehabilitation therapy, they were administered Imupret®, the commercial herbal medicinal product (Bionorica AG, Neumarkt, Germany) and an immunocorrective and antiphlogistic agent, 25 drops four times daily for two weeks. In addition, Eebisol, an IFN-? inducer and repairing agent, was administered intramuscularly 2 ml in the evening for 20 days. Moreover, patients received one tablet of Aerius (Desloratadine), an antihistamine agent, at bed time for 10 days. They received also liposoluble A and E vitamins, AEvit (Kyiv Vitamin Factory JSC, Kyiv, Ukraine), 1-2 capsules a day for 20 days, and D3 vitamin, Ergocalciferol (Lekkhim, Poland), at a prophylactic dose. Patients underwent re-examination one week after completing their rehabilitation treatment. Ten age-matched patients were included in the study as controls and also underwent examination. Blood samples were taken from the cubital vein; serum was separated and preserved at -20o C until assays were run. Serum immunoglobulin IgM and IgE levels were measured using enzyme-linked immunosorbent assays (ELISA) from Xema-Medica Ltd (Russia) and Lab line reader (Austria). In addition, serum levels of interleukin (IL)-1? (proinflammatory cytokine), IL-10 (anti-inflammatory cytokine), gamma interferon [TH1(IFN-?)], and transforming growth factor (TGF)-1? were measured with ELISA kits from Tsitokin Ltd (St Petersburg, Russia) and Vektor-Best (Novosibirsk, Russia). Biostatistika-6 software was used to perform statistical analysis with the non-parametric Wilcoxon U test and Student t-test as per Gubler (Gubler, 1990). Results and Discussion There was no significant difference in serum IgM and IgE levels between either group of FBT patients and controls (Figs. 1, 2).
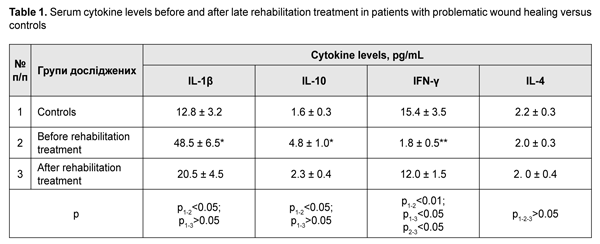
Table 1 presents serum levels of proinflammatory IL1? and IFN-? and anti-inflammatory IL-10 and IL-4 cytokines.
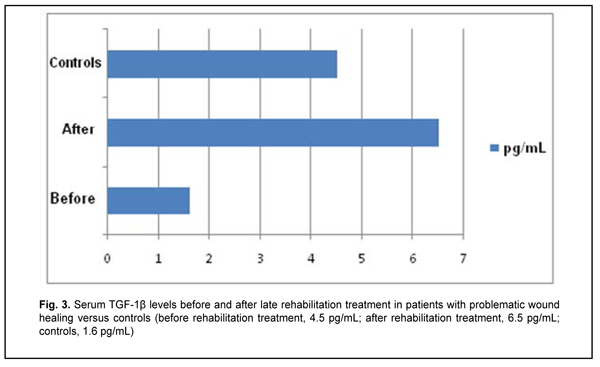
Late after treatment of FBT, serum IL1? and IL-10 levels in patients with a problematic course were still higher compared to controls (Table 1). In addition, serum level of IFN-?, a key immunity factor, significantly decreased, but no change was found in serum level of IL-4 in patients with a problematic course. Therapeutic immune rehabilitation measures significantly improved cytokine levels and coincided with improvements in clinical status in this category of patients with FBT. Prior to immune rehabilitation treatment, FBT patients with a problematic course exhibited decreased serum TGF-1? levels, indirectly indicating low repair process activity (Drannik, 2010) and low T-cell activity. Serum TGF-1? levels in FBT patients with a problematic course tended to increase after immune pharmacotherapy (Fig. 3), which was beneficial for repair process activity (Holm, 2004).
Therefore, we (1) studied FTB patients who exhibited either uneventful or eventful clinical recovery after surgery involving the use of the biocomposite, and (2) found abnormalities in immunity characteristics (like activation of pro- and anti-inflammatory interleukins) in patients exhibiting an eventful recovery; such abnormalities are considered as a manifestation of a prolonged recovery process (Melnikov et al, 2015; Matricon et al, 2010). Since a significantly decreased blood level of a key immunity factor, IFN-?, has a pathogenetic role (Demianov, Kotov, Simbirtsev, 2003; Drannik, 2010), the use of interferon inducers as an adjunct to therapy in FBT patients with a problematic recovery course might be reasonable. A weakened normalization process in patients with a problematic recovery course can be explained also by low levels of growth factors, particularly, TGF-1?, that plays a role in regenerative processes in most tissues of the body. Immune pharmacotherapy contributed to improvements in immunity characteristics and clinical status of patients with a problematic postoperative course. Conclusion First, after surgery for facial bone trauma, some patients exhibit prolonged recovery and abnormal production of cytokines, reagins and regulatory proteins, which requires for immunocorrective therapy. Second, the rehabilitation treatment regimen involving an immune modulating agent, IFN-? inducer, antihistamine agent and liposoluble vitamins facilitated improvements in immunity characteristics and clinical status of patients with a problematic postoperative course.
References 1.Gorbunov VI, Likhterman LV, Gannushkina IV. [Immunology of traumatic brain injury]. Ulianovsk:SVNTS; 1996. Russian. 2.Gubler ЕV. [Informatics in pathology, clinical medicine and pediatrics]. Leningrad:Meditsina; 1990. Russian. 3.Demianov AV, Kotov AIu, Simbirtsev AG. [Diagnostic value of studying cytokine levels in clinical practice]. Cytokines and inflammation. 2003;3:20–8. Russian. 4.Drannik GN. [Clinical immunology and allergology]. Kyiv:Polygraph plus; 2010. Russian. 5.Kishchuk VV, Bondarchuk OD. [Using Syntekist biocomposites to repair bone defects in ear, nose and throat organs]. Zhurnal vushnykh, nosovykh i gorlovykh khvorob. 2007;5c:43–4. Ukrainian. 6.Kishchuk VV, Bondarchuk OD, Dmytrenko IV, Lobko KA. [Changes in humoral systemic immunity as an objective criterion of the rehabilitation process in patients with frontal basilar trauma]. Zhurnal vushnykh, nosovykh i gorlovykh khvorob. 2018;5:45. Ukrainian. 7.Kovalchuk LV, Gankovskaya LV, Meshkova RY. [Clinical immunology and allergology with the basics of general immunology]. Moscow: GEOTAR–Media; 2011. Russian. 8.Lysianyi NI, Pedachenko EG, Kadzhaia NV. [Features of autoimmune responses developing in recurrent TBI]. Immunologiia ta allergologiia. 2006;3:53–6. Ukrainian. 9.Nasonov EL. [Methodological aspects of measurements of circulating immune complexes with the use of polyethylene glycol]. Terapevticheskii arkhive. 1987;4:38–45. Russian. 10.Iakovenko VD. [Experimental and clinical grounding of the allergic diagnosis of chronical tonsillitis]. [Abstract of Cand Sc (Med) Thesis]. Kyiv: Prof. Kolomiichenko Institute of Otolaryngology; 1985. Russian. 11.Kabat EA, Mayer MM. [Experimental immunochemistry]. Moscow:Mir; 1968. Russian. 12.Matricon J, Barnish N, Ardid D. [Immunopathogenesis of inflammatory bowel disease]. Med Sci (Paris). 2010 Apr;26(4):405-10. French. 13.Zhuang J, Shackford SR, Schmoker JD, Anderson ML. The association of leukocytes with secondary brain injury. J Trauma. 1993 Sep;35(3):415-22.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|