J.ophthalmol.(Ukraine).2020;4:8-13.
|
http://doi.org/10.31288/oftalmolzh20204813 Received: 18 May 2020; Published on-line: 27 August 2020 Orbital US-based characteristics in Graves’ disease-associated autoimmune ophthalmopathy Yu.V. Buldygina, G.M. Terekhova, Ie.A. Shelkovoy, T.V. Fed’ko Komisarenko Institute for Endocrinology and Metabolism, NAMS of Ukraine; Kyiv (Ukraine) E-mail: Yuliya.buldygina@icloud.com TO CITE THIS ARTICLE: Buldygina YuV, Terekhova GM, Shelkovoy IeA, Fed’ko TV. Orbital US-based characteristics in Graves’ disease-associated autoimmune ophthalmopathy. J.ophthalmol.(Ukraine).2020;4:8-13.http://doi.org/10.31288/oftalmolzh20204813 Background: Autoimmune ophthalmopathy (AO) may develop in as much as 90% patients with Graves’ disease (GD), and may lead to visual disability. Adequate imaging of orbital structures allows planning adequate treatment in most cases, contributing to better treatment outcomes in patients with AO. Purpose: To examine orbital ultrasound (US)-based characteristics in patients with GD-associated AO. Material and Methods: One hundred patients with GD-associated AO underwent a comprehensive examination that included (1) determination of AO activity by clinical activity score (CAS), (2) measurements of thyrotropin (TSH), free thyroxine (FT4), free triiodothyronine (FT3), and TSH-R-Ab levels; and (3) US of the thyroid gland and orbital tissue. In addition, 31 of these patients underwent magnetic resonance imaging (MRI). The Student t test was used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± SEM. Results: One hundred patients (73 women and 27 men; age, 18 to 79 years; mean age, 48.42 ± 13.56 years; mean disease duration, 3.1 ± 2.2 years) with GD-associated AO were included in this study. Of these, 65% were euthyroids, whereas 18% and 17% were found to have clinical hyperthyroidism and subclinical hyperthyroidism, respectively. As expected, the mean TSH-R-Ab level (11.07±1.03 U/mL) was higher than normal (<0.55 U/mL), confirming the presence of GD. Most patients had class III (36%) or class IV (34%) disease according to NO SPECS classification, 49 patients had a CAS score > 3 (active disease), and 51 had a CAS score < 3 (non-active disease). Orbital US found that, in all patients, the lens was normally located anatomically, and the chorioretinal complex and the subdural space of the optic nerve were of normal thickness. Retrobulbar adipose tissue (RAT) edema was found in 49 patients with active disease. Of these 49, four also exhibited eyelid edema. RAT fibrosis without muscle fibrosis was found in 21 patients, and with muscle fibrosis, in 30 patients. Although 49 patients had active AO, US-based thickness of the orbital rectus muscles was within reference intervals. In all patients, the rectus muscles were seen as hypoechogenic structures. There was a significant difference in orbital rectus muscle thickness measurements between orbital US and MRI. Thus, MRI-based thickness of medial, lateral, superior and inferior rectus muscles was significantly greater (р < 0.05) than US-based thickness of these muscles, with US demonstrating normal thickness. Conclusion: First, orbital ultrasonography found RAT edema in 49%, and RAT fibrosis in 51% of patients with GD-associated AO. Second, in all patients with active AO, orbital US provided valuable data for assessing the status of RAT and determining whether RAT edema or fibrosis was present. Third, MRI-based thickness of orbital rectus muscles was significantly (p < 0.05) greater than US-based thickness of these muscles, with US demonstrating normal thickness. Finally, because orbital US does not provide reliable data for determining orbital rectus muscle thickness, especially in active AO, it is reasonable to use orbital MRI for this purpose. Keywords: autoimmune ophthalmopathy, Graves’ disease, ultrasound examination, magnetic resonance imaging
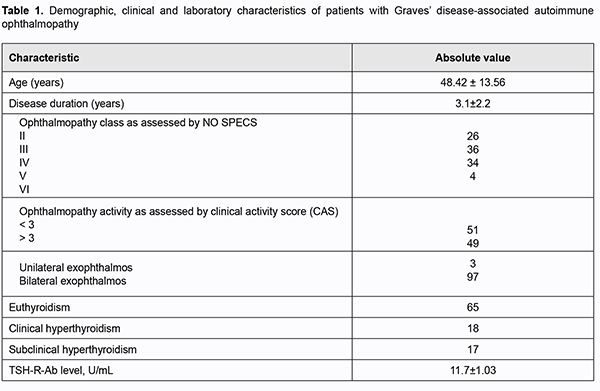
Introduction Autoimmune ophthalmopathy (AO; or Graves’ ophthalmopathy) may be associated with Graves’ disease (GD) in as much as 90% of cases, is characterized by a complex orbital tissue injury and accompanied by infiltration, edema and the proliferation of retrobulbar adipose tissue (RAT), muscles and connective tissue. The pathogeneses of AO is not fully elucidated. In AO, orbital fibroblasts are the autoimmune target cells and express relatively high levels of functional thyroid-stimulating hormone receptor (TSH-R). Stimulating antibodies (Ab) bind to TSH-R that also interacts with insulin-like growth factor 1 receptors (IGF1R) on the surface of thyrocytes and on orbital fibroblasts, with the TSH-R-Ab interaction with TSH-R activating both IGF1R downstream pathways and TSH-R signaling [1, 2]. Clinical studies (disease activity assessment by clinical activity score (CAS), hormone level and immunological studies, orbital ultrasound (US) and magnetic resonance imaging (MRI)) are important for the diagnosis of GD-associated AO. It has been demonstrated that disease activity assessment by CAS was helpful in predicting treatment response [3, 4, 5, 6], especially if the activity was also assessed by US [4, 7, 8]. Orbital US is a widely available and effective technique for diagnosing AO which allows discriminating between orbital edema and fibrosis, and in this way determining more precisely whether inflammation is active [5, 9, 10]. Ultrasound findings of edema or fibrotic changes in RAT and orbital rectus muscles are major ultrasonographic criteria for assessing the status of patients with AO. Patients with clinically manifest AO commonly exhibit asymmetric exophthalmos with eyelid edema that is easily identified on US as hypodermal thickening. RAT edema results in an increased distance between the posterior pole of the eye and the orbital apex. In the presence of edema, RAT has increased and somewhat heterogeneously dispersed echogenicity. With progression of the disease, RAT becomes seen as a heterogenous echogenic structure due to the presence of diffuse hyperechogenic inclusions attributed to fibrous changes in the tissue. Edema of orbital rectus muscles in AO is sonographically seen as spindle-shaped enlargement of the rectus muscles with homogeneously decreased echogenicity. Medial rectus muscles are the first to become enlarged. Subsequently, fibrosis and fatty degeneration of rectus muscle fibers take place, with a degenerated muscle seen as a homogeneous highly echogenic structure. Such changes in muscle echogenicity may complicate the identification of muscle tissue against the background of adipose tissue [11, 12]. There are two major disadvantages of orbital US compared with MRI. The first is a low accuracy of muscle volume assessment; this is attributed to fibrosis, which is commonly associated with AO and complicates muscle delineation [8, 10, 13], and to the fact that MRI allows identifying the thickest portion of the muscle, whereas US is limited by the viewing angle [14]. The second is that, compared with MRI, orbital ultrasonography is believed to be less accurate with regard to visualization of the posterior orbit [13] and bone architecture. Most researchers are in agreement that US and MRI cannot substitute each other, but, from the clinical point of view, the two techniques, when used in combination, may be helpful in a comprehensive assessment of the complex health condition. Practitioners use orbital US more frequently than MRI of the orbit, because, unfortunately, the latter technique is more expensive and thus less available than the former; however, orbital US may be also used for assessing the progression or regression of ophthalmopathy [14]. The purpose of this study was to examine orbital ultrasound-based characteristics in patients with Graves’ disease-associated autoimmune ophthalmopathy. Material and Methods This study included investigation of the course of autoimmune ophthalmopathy on the basis of orbital US images. A comprehensive examination included (1) examination by an ophthalmologist to classify clinical severity of AO using the NO SPECS and to assess disease activity by CAS, (2) measurements of thyrotropin (TSH), free thyroxine (FT4), free triiodothyronine (FT3), and TSH-R-Ab levels; and (3) ultrasonography of the thyroid gland and orbital tissue. Werner's classification [15] of the eye changes of Graves’ disease divides ocular symptoms and signs into classes from 0 to 6, and we used this classification to assess the severity of the disease course. The CAS [3, 4] is calculated by assigning one point to each manifestation (spontaneous retrobulbar pain; pain on attempted upward or downward gaze; redness of eyelids; redness of conjunctiva; swelling of caruncle or plica; swelling of eyelids; and swelling of conjunctiva (chemosis)) and summing the points. Patients with a CAS ≥ 3 were considered as having active disease. Measurements of TSH, FT4, and FT3 were conducted at the laboratory of Radionuclide Diagnostics and Therapy Department of the Komisarenko Institute using chemiluminescent immunoassay kits (reference TSH range, 0.27-4.2 μIU/mL; reference FT4 range, 11.97-22.01 pMol/L; reference FT3 range, 2.02-4.43 pg/mL) on Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Measurements of TSH-R-Ab were conducted using chemiluminescent immunoassay kits on a Siemens Immulite 2000 analyzer. Thyroid antibody titers were considered positive for the anti-TSH receptor-Ab titers above 0.55 U/L. Orbital US was performed using NEMIO SSA-550A system (Toshiba Medical Systems Co, Ltd, Tokyo, Japan) and NEMIO SSA-580A system (Toshiba) equipped with 8–14 MHz linear probes to assess echogenicity of the lens and vitreous, and thickness of the chorioretinal complex, retrobulbar adipose tissue, optic nerve subdural space, and medial and lateral, superior and inferior orbital rectus muscles. Orbital MRI was performed using a 0.4-T machine (Aperto Permanent Magnet; Hitachi, Tokyo, Japan) in STIR mode. In patients with active AO, thickness of the orbital rectus muscles assessed with MRI was compared with that obtained with orbital US. It has been reported that normal ranges for thickness of medial rectus muscle, inferior rectus muscle, superior rectus muscle, and lateral rectus muscle were 3.2-4.9 mm, 3.0-6.0 mm, 3.1-5.6 mm, and 2.6-4.8 mm, respectively [16]. The Student t test was used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± SEM. Results and Discussion One hundred patients (73 women and 27 men; age, 18 to 79 years; mean age, 48.42 ± 13.56 years) with GD-associated AO were included in this study. Disease duration ranged from 4 months to 7.6 years (mean duration, 3.1 ± 2.2 years). Table 1 presents clinical characteristics and laboratory data for patients.
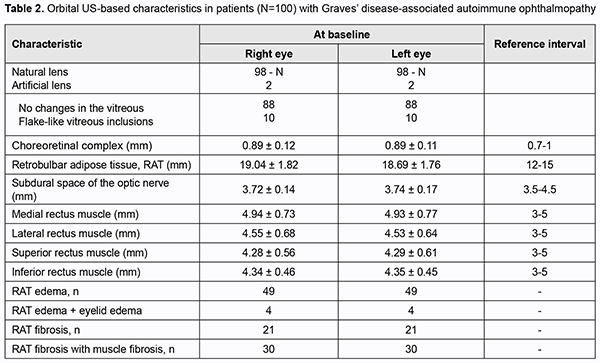
Most commonly, patients were middle-age euthyroid women with GD-associated AO. In addition, clinical examination of the orbits found that most patients had class III (36%) or class IV (34%) disease according to NO SPECS classification, 49 patients had a CAS score > 3 (active disease), and 51 had a CAS score < 3 (non-active disease). As expected, the mean TSH-R-Ab level (11.07±1.03 U/mL) was higher than normal (<0.55 U/mL), confirming the presence of Graves’ disease. Results of orbit US for 100 patients of the study are summarized in Table 2. In all patients, the lens was normally located anatomically, and the chorioretinal complex and the subdural space of the optic nerve were of normal thickness. RAT edema was found in 49 patients with active disease. Of these 49, four also exhibited eyelid edema. RAT fibrosis without muscle fibrosis was found in 21 patients, and with muscle fibrosis, in 30 patients. Although 49 patients had active disease, US-based rectus muscle thickness was within reference intervals. Of note, in all patients, the rectus muscles were seen as hypoechogenic structures.
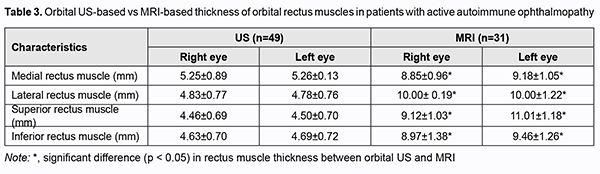
We also compared RAT thickness and rectus muscle thickness measured by ultrasonography vs MRI for patients with active disease (49 patients; 38 women and 11 men) (Table 3). There was a significant difference in orbital rectus muscle thickness measurements between ultrasonography and MRI. Thus, MRI-based thickness of medial, lateral, superior and inferior rectus muscles was significantly greater than US-based thickness of these muscles, with US demonstrating normal thickness. Therefore, US was found to be accurate for measuring RAT thickness, but not orbital rectus muscle thickness, for which MRI is more advantageous than US.
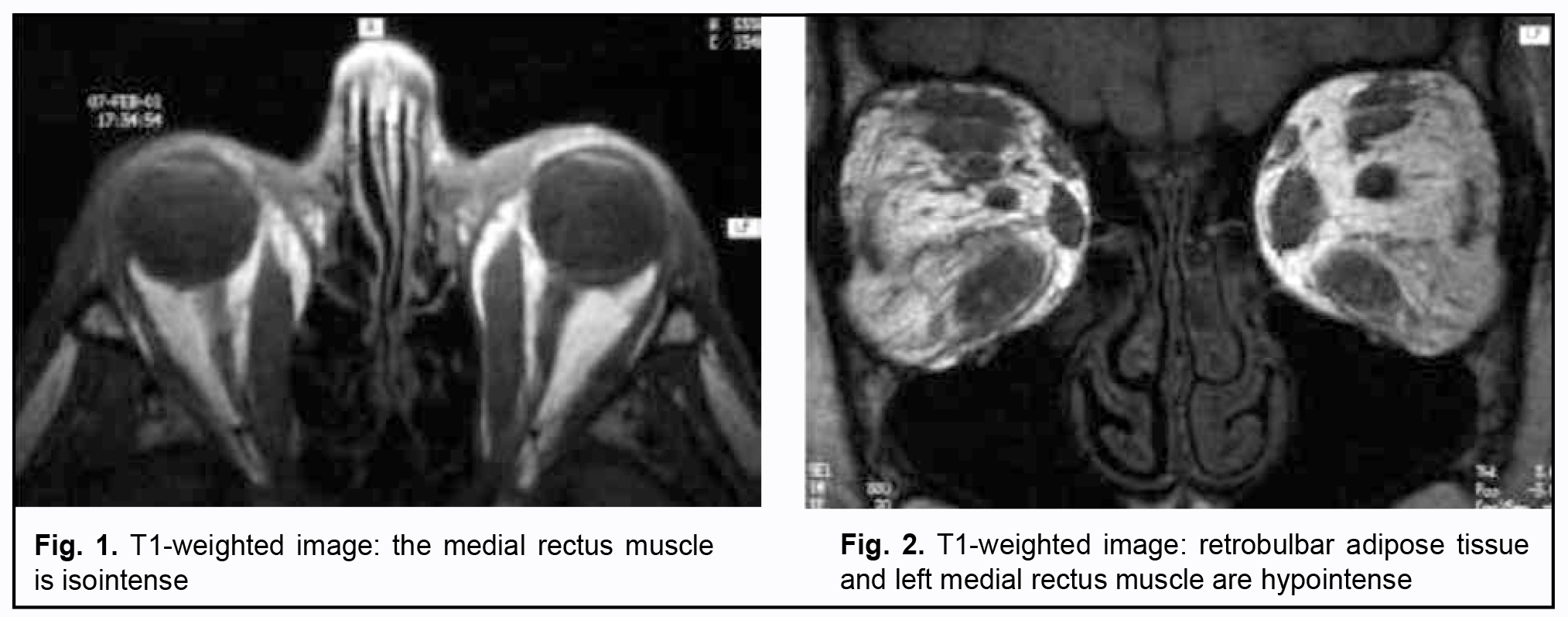
Figs 1 and 2 [17] show orbital MRI images and exemplify the findings.
Therefore, this study allowed assessing the potential of orbital US in the diagnosis of AO. The orbital US was helpful for determining RAT thickness and whether RAT edema or fibrosis was present, and assessing the status of the lens, chorioretinal complex, and subdural space of the optic nerve. The relevant findings of orbital ultrasonography were in agreement with clinical manifestations of disease and disease activity as measured by CAS. They were also consistent with reports of others [3, 4, 5, 6, 7, 8] who demonstrated the efficacy of US for detecting pathological changes in the orbit in AO. However, US-based orbital muscle thickness was normal, although in active disease, orbital muscle thickness had to be greater than normal. Subsequently, patients with active AO underwent MRI, and the results of MRI were compared with orbital US. There was a significant difference (p < 0.05) in orbital rectus muscle thickness measurements between US and MRI. MRI-based thickness of medial, lateral, superior and inferior rectus muscles were significantly greater than US-based thickness of these muscles, with ultrasonography demonstrating normal thickness. This finding is consistent with reports of others [8, 10, 13, 14], who also demonstrated a significant difference in orbital rectus muscle thickness measurements between US and MRI. Therefore, orbital US is a technique that provides valuable data for assessing the status of RAT and determining whether RAT edema or fibrosis is present in AO, thus availing an opportunity to redefine treatment strategy. In addition, the technique may provide valuable data for differential diagnosis and monitoring the efficacy of treatment of AO. However, because orbital US does not provide reliable data for determining orbital rectus muscle thickness, especially in active AO, it is reasonable to use orbital MRI for this purpose. In conclusion, first, orbital US found RAT edema in 49%, and RAT fibrosis in 51% of patients with in Graves’ disease- associated autoimmune ophthalmopathy. Second, in all patients with active AO, orbital US provided valuable data for assessing the status of RAT and determining whether RAT edema or fibrosis was present. Third, MRI-based thickness of orbital rectus muscles was significantly (p < 0.05) greater than US-based thickness of these muscles, with US demonstrating normal thickness. Finally, because orbital US does not provide reliable data for determining orbital rectus muscle thickness, especially in active AO, it is reasonable to use orbital MRI for this purpose.
References 1.Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves' Hyperthyroidism. Eur Thyroid J. 2018 Aug;7(4):167-86. 2.Smith TJ, Janssen JAMJL. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr Rev. 2019 Feb 1;40(1):236-67. 3.Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000 Apr;21(2):168-99. 4.Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989 Aug;73(8):639-44. 5.Gerding MN, Prummel MF, Wiersinga WM. Assessment of disease activity in Graves' ophthalmopathy by orbital ultrasonography and clinical parameters. Clin Endocrinol (Oxf). 2000 May;52(5):641-6. 6.Stamato FJ, Maciel RM, Manso PG, Wolosker AM, Paiva ER, Lopes AC, Furlanetto RP. Colchicine in the treatment of the inflammatory phase of Graves' ophthalmopathy: a prospective and randomized trial with prednisone. Arq Bras Oftalmol. 2006 Nov-Dec;69(6):811-6. 7.Prummel MF. Graves' ophthalmopathy: diagnosis and management. Eur J Nucl Med. 2000 Apr;27(4):373-6. 8.Kahaly GJ. Imaging in thyroid-associated orbitopathy. Eur J Endocrinol. 2001 Aug;145(2):107-18. 9.Erickson BA, Harris GJ, Lewandowski MF, Murray KJ, Massaro BM. Echographic monitoring of response of extraocular muscles to irradiation in Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 1995 Feb1;31(3):651-60. 10.Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B. Graves' ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound. 2005 Oct;33(8):375-80. 11.Zubarev AV, editor. [A practical guide to diagnostic ultrasound in ophthalmology]. Moscow: Strom; 2002. Russian. 12.DiBernardo CW, Greenberg E. Ophthalmic Ultrasound: A Diagnostic Atlas. New York: Thieme Medical Publishers; 2007. 13.Nagy EV, Toth J, Kaldi I, Damjanovich J, Mezosi E, LenkeyA, et al. Graves’ ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000 Jun;142 (6): 591–7 14.Gorman CA. The measurement of change in Graves' ophthalmopathy. Thyroid. 1998 Jun;8(6):539-43. 15.Werner SC. Classification of the eye changes of Graves' disease. Am J Ophthalmol. 1969;68(4):646–8. 16.Ozgen A, Aydingöz U. Normative measurements of orbital structures using MRI. J Comput Assist Tomogr. 2000 May-Jun;24(3):493-6. 17.Zhou Quan. Die Rolle der orbitalen MRT in der Differentialdiagnose von Erkrankungen der Augenmuskeln, des extrakonalen und subperiostalen Kompartimentes. Dissertation. 2003. Germany. 96 p. DOI: 10.18452/14831. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|