J.ophthalmol.(Ukraine).2020;4:28-32.
|
http://doi.org/10.31288/oftalmolzh202042832 Received: 27 March 2020; Published on-line: 27 August 2020 A New Parameter in The Etiology of Hyperopic Anisometropic Amblyopia: Corneal Densitometry Erdin? Bozkurt, Assist. Prof.; Ersin Muhaf?z, Assist. Prof.; Hayrunisa Bekis Bozkurt , Assist. Prof.; Serif Nizamo?lu, Asistant Kafkas University, Faculty of Medicine; Kars (Turkey) E-mail: drerdincbozkurt@hotmail.com TO CITE THIS ARTICLE: Erdin? Bozkurt, Ersin Muhaf?z, Hayrunisa Bekis Bozkurt, Serif Nizamo?lu. A New Parameter in The Etiology of Hyperopic Anisometropic Amblyopia: Corneal Densitometry. J.ophthalmol.(Ukraine).2020;4:28-32. http://doi.org/10.31288/oftalmolzh202042832 Objectives. To evaluate the anterior segment parameters and densitometric values of cornea and lens in children with anisohipermetropic amblyopia by corneal topography and optical biometry devices. Design. Prospective cross sectional study. Participants. Ambliopic and fellow eyes of 42 children with anisohipermetropic amblyopia (19 males, 23 females, mean age 9.98±3.88 years) and right eyes of 44 healty children from pediatric outpatient (22 males, 22 females, mean age 9.41±4.1 years) were included in this study. Methods. All patients were evaluated with sirius topography and optical biometry. Visual acuities, spherical refractive values, mean keratometric values, axial lengths, central corneal thickness, corneal volume, anterior chamber depth, anterior chamber volume, iridocorneal angle, pupil diameter, white to white, cornea densitometry and lens densitometry of amblyopic eyes, fellow eyes and healthy eyes were compared. Results. Visual acuity was 0.332±0.024, 0.0132±0.0058, 0.001 (LogMAR) for amblyopic, fellow and healthy eyes respectively (p = 0.003), spherical refractive 3.79±2.52, 1.33±1.29 and 0.98±0.39 respectively (p = 0.025). ICA and central corneal densitometry were found lower in amblyopic eyes than in fellow and healty eyes among sirius corneal topography parameters (p value 0.041, 0.001). There was no significant difference between groups in terms of optical biometry parameters except axial length. Conclusion. In our study, we found that there were significant differences in iridocorneal angle, central corneal densitometry between groups. We think that it may be an important parameter in corneal density except for spherical equivalent and axial length in the etiology of hyperopic anisometropic amblyopia. Keywords: аnisohipermetropic amblyopia, anterior segment parameters, cornea and lens densitometry, corneal topography, optical biometry
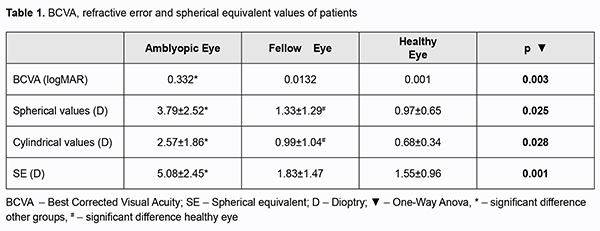
Amblyopia is defined as a decrease in visual acuity resulting from abnormal binocular interaction, which can be improved with treatment during visual development, unilateral or bilateral visual acuity. In the researches, amblyopia was the most common cause of unilateral vision loss [1]. In the studies on the prevalence of amblyopia, rates ranging from 1% to 5.4% were found in different age groups [2-3] The study of the prevalence of amblyopia in Turkey, Eski?ehir, Istanbul, Ankara and Southeastern Anatolia Region prevalence was found to be between 2.6% and 5.5% [4]. Strabismus, anisometropia, visual deprivation and uncorrected refraction defects are accused in the etiology of amblyopia [1-5]. In anisometropic amblyopia, when there is more difference than 2 diopters (D) between the two eyes, amblyopia develops as a result of blurring of the retinal imagination in the eye with high refractive error, and suppression of the stimuli from the eye with shift in strabismic amblyopia. While clinical conditions such as congenital cataract, corneal opacity, complete ptosis and edema, inflammation, hemangioma, and closure of the eyelid cause visual deprivation in deprivation amblyopia, amblyopia may develop as a result of failure of visual system if no optical correction is made in high grade refraction defects [5]. Although the pathophysiological mechanism of anisometropic amblyopia cannot be fully explained, sensory discrepancy caused by the difference in the image in the retinal layer of both eyes is accused [5]. There is no other study in the literature in which the anterior segment parameters of anisohipermetropic cases in the amblyopia, fellow and healthy eyes were evaluated with corneal topography (especially cornea and lens densitometry) and optical biometry. In this respect, this study may be the first in the literature. In this study, to investigate the anterior segment parameters and densitometric values of cornea and lens in children with anisohipermetropic amblyopia by corneal topography and optical biometry devices. Methods This study is a prospective, cross-sectional study. In accordance with the Declaration of Helsinki and ethics approval was obtained from the local ethics committee with the number 80576354-050-99 / 43. Consent was obtained from the guardians of the participants included in this study. Ambliopic and fellow eyes of 42 children with anisohipermetropic amblyopia from ophthalmology department and right eyes of 44 healty children from pediatrics department were evaluated by sirius topography and optical biometry. Amblyopia criterion was accepted as the best corrected visual acuity of 0.8 or less with Snellen chart and at least 2 lines difference between both eyes. Anisometropia was defined as a cylindrical refraction difference of at least 1.00 D and above and a spherical refraction difference of 2 D and above between the two eyes. Spherical equivalent was calculated as the sum of the spherical value and half of the cylindrical value. Patients with visual acuity less than 1 according to LogMAR chart and who cannot fixate and children under five years of age who may have difficulty in adaptation to the devices, patients with organic pathology, nystagmus and a shift above 10 D, who have undergone eye surgery or corneal trauma was excluded. Detailed ophthalmologic examination was performed in all cases included in our study. The best corrected visual acuity (BCVA) and the logarithmic responses of the minimum resolution angle (LogMAR) of these values were determined. Anterior segment and fundus examination findings were recorded in all cases. Refraction defects were determined by autorefractokeratometer (Topcon KR-8100) 45 minutes after instillation of cyclopentolate hydrochloride 1% (Cycloplegine 1%, Abdi ?brahim Pharmaceutical Industry and Trade Inc.) The treatment of the ambliopic patients in our study was the application of glasses to correct the refraction error and the age-related patch (daily closure per hour equivalent to age). Cycloplegic autorefractokeratometry of all cases included in the study was measured by same person. Mean keratometry, corneal thickness map and central corneal thickness, anterior chamber depth, anterior chamber angle and corneal volumes, pupil diameter, densitometry of the center, 2 mm nasal and temporal sections of the cornea, central lens densitometry was measured with corneal topography device (Sirius, CSO, Florence, Italy). The Sirius corneal topography device is able to scan more than 30,000 points on the anterior and posterior surface of the cornea and the cornea and anterior chamber with 25 radial sections at the same time thanks to the 360° rotatable Scheimpflug camera system and the 22-ring plasido disc [6]. Axial lenghts, central corneal thickness, anterior chamber depth, pupil diameter, white to white diameter were recorded by optical biometry. The Nidek AL-Scan optical biometry device (Nidek, Aichi, Japan) performs axial length measurement using partial coherence laser interferometry technology. It calculates the refractive power of the cornea by detecting the straightest and steepest meridians with the help of photodetector by creating a ring image projection on the cornea of the patient [7]. Statistical analysis was performed with SPSS (Statistical Package for Scientific Studies, Windows version 21.0; SPSS Inc., Chicago, USA) 21.0 statistical package program with 95% confidence. While evaluating the study data, descriptive statistics; mean, standard deviation, median, frequency, ratio, minimum, maximum were used. Paired samples t and One-Way Anova test were used for the analysis of the quantitative data. Statistical significance was accepted as p<0.05. Results Forty-two children with anisohipermetropic amblyopia (19 males, 23 females, mean age 9.98±3.88 years) and 44 healty children (22 males, 22 females, mean age 9.41±4.1 years) from pediatric outpatient were included in the study. There was no significant difference between the groups in terms of age and gender (p values 0.286, 0.195 respectively). Visual acuity, spherical and cylindrical refractive value, spherical equivalent were shown in table 1. All parameters were significant between the groups (p value 0.003, 0.025, 0.028, 0.001 respectively).
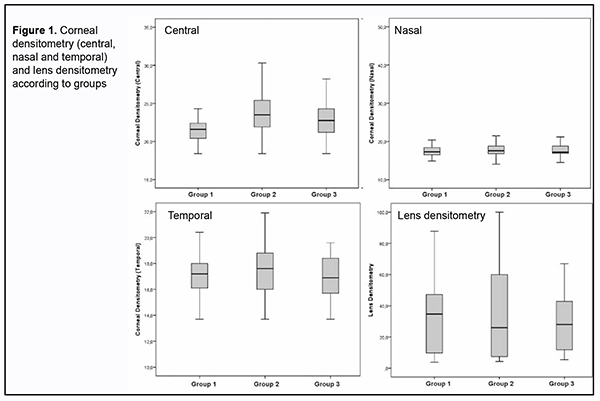
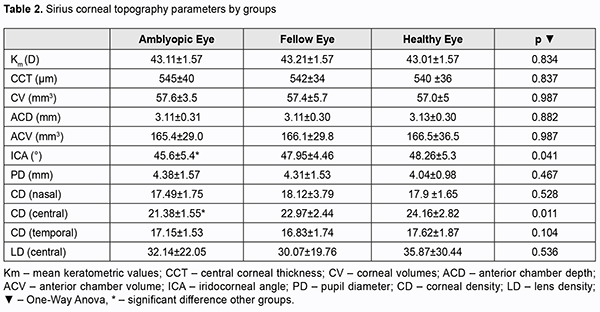
Sirius topography parameters for amblyopia, fellow and healthy eyes were shown in table 2. There was significant difference between the groups in terms of central corneal densitometry and ICA (p value 0.011, 0.041 respectively).
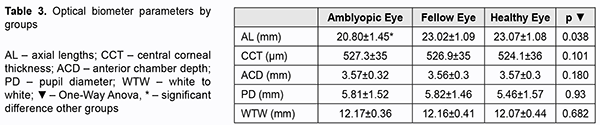
AL-Scan optical biometry parameters for the amblyopia, fellow and healthy eye were shown in table 3. There was no significant difference between the groups in terms of CCT, ACD, PD and WTW (p value 0.038, 0.101, 0.180, 0.93, 0.682 respectively), except AL (p=0.038). In 71.4% of amblyopic eyes, the axial length measured by optical biometry was shorter than the average of the axial length of the fellow and healthy eyes.
D?scuss?on Amblyopia; it is used to describe the poor visual acuity caused by abnormal visual development during the critical period in childhood and is characterized by a loss of visual acuity ranging from not seeing a few letters in the range of 1.0 or 60/60 to hand movements. Uncorrected refractive errors in the pediatric age group may lead to amblyopia in contrast to adults. If the amblyopia developed in relation to the visual development process is not treated in early childhood, vision loss may be permanent and treatment in adulthood is not possible [8-9]. It is important to examine the factors that can cause this condition which cannot be treated in elderly. Therefore, the effect of anterior segment parameters was invastigated in amblyopia patients. Anisometropy is defined as a spherical difference of 2 D and above or a cylindrical difference of 1D and above between eyes [10]. It has been reported that 1D difference for spherical anisometropy and 1.5D difference for cylindrical anisometropia significantly increase the risk of amblyopia [11]. The difference in anisometropia up to 2.5D detected in infantile or early childhood decreases during the emmetropization process and does not cause amblyopia [12]. Therefore, anisometropic refraction defects, especially after 4 years of age, carry a great risk for amblyopia and close follow-up of these cases is required. In this study, biometric and topographic parameters were investigated along with refractive changes causing anisometropia and the variables were tried to determine between the groups. There was a significant difference between spheric, cylindrical and spherical equivalent values measured by autorefractokeratometer between the groups. Axial lenghts measured by optical biometry was found to be significantly shorter in amblyopic eyes. In 71.4% of amblyopic eyes, the axial length measured by optical biometry was shorter than the average of the axial length of the fellow and healthy eyes. This shows that the short axial length is the major risk factor in amblyopia in terms of biometric parameters. Axial hyperopia has been accused as the cause of amblyopia in anisometropia in several studies [13-14]. This study suggests that not only axial hyperopia, corneal spherical equivalent but also corneal densitometry may play a role in the etiology of anisohipermetropic amblyopia. In our study, no significant difference was found between the groups in terms of mean keratometric, central corneal thickness, corneal volume, anterior chamber depht, anterior chamber volume, pupil diameter, temporal corneal density and lens density parameters measured by corneal topography. Wang et al. similar to this study, in hyperopic anisometropic amblyopia, no significant difference was observed between amblyopia and healthy eyes in terms of anterior segment parameters [15]. Y?ksel et al. evaluated the anterior segment parameters of children with amblyopia by pentacam and no significant difference was observed between these parameters [16]. However, these two studies do not provide information about corneal and lens densitometry of children with amblyopia. In our study there was stastistical significant difference between groups in recpect of corneal densitometry. Also, spherical equivalent, axial lenght and iridocorneal angle were different between the groups. In the light of these findings, these may play a role in the etiology of amblyopia. Unlike this study, there are studies indicating that anterior chamber depht may be effective on amblyopia [17]. As far as is known, corneal and lens densitometry has not been investigated in the patients with amblyopia. In this respect, this study is the first in the literature. Corneal and lens density is an indicator of transparency. Lopes et al. reported that keratoconic corneas increased densitometry compared to normal corneas and increased density in advanced keratoconus in patients with keratoconus [18]. Ahmed et al. investigated corneal density for healing of patients with bacterial keratitis, reported that active infected infiltrates increased densitometry relative to the scar area of the cornea [19]. There are many studies on lens densitometry using Scheimpflug imaging [20]. It is stated that lens densitometry increases especially with age [21]. It has been reported that lens density increases with increasing degree of cataract, which decreases the optical quality of the eye [22]. However, a study about lens densitometry has not been found in the patients with amblyopia in the literature. In our study, there was no significant difference between lens densitometry values between the groups (p=0.536). In this study, axial lenght, central corneal thickness, anterior chamber depht, pupil diameter, white to white parameters were compared amblyopia, fellow and healthy eyes measured by optical biometry. There was no significant difference between the values except axial lenght. In the study of Huang et al. with the optical biometry device, the reproducibility and reliability of AL-Scan were excellent for all parameters except pupil diameter, white to white parameters [23]. It has shown that the results obtained from optical biometry data show excellent sensitivity and specificity to detect amliopic refractive risk [24]. In a study performed in myopic anisometropic eyes, it was stated that there was no significant change in anterior segment parameters compared to the other eye [25]. In addition, in this study, no significant difference was found between the results of both devices in terms of central corneal thickness, anterior chamber depht and pupil diameter values. The fact that the results of both devices are close to each other indicates that the results are reliable. Limitation of study: Comparison of hypermetropic amblyopic and non-ambliyopic eyes in terms of ICA and corneal densitometry may show the strenght of these parameters in amblyopia etiology. Conclus?on In this study, we found that there were significant differences in spherical equivalent, axial hyperopia, iridocorneal angle and central corneal densitometry between groups. We think that it may be an important parameter in corneal density except for spherical equivalent and axial length in the etiology of hyperopic anisometropic amblyopia.
References 1.Holmes JM, Clarke MP. Amblyopia. Lancet. 2006; 22: 1343-51. 2.Hatt S, Antonio-Santos A, Powell C et al. Interventions for stimulus deprivation amblyopia. Cochrane Database Syst Rev. 2006; 19: 3. 3.Flom MC. Neumaier R.W. Prevalence of Amblyopia. Public Health Reports 1966; 81: 329-341. 4.Caca I, Cingu AK, Sahin A et al. Amblyopia and refractive errors among school-aged children with low socioeconomic status in southeastern Turkey. J Pediatr Ophthalmol Strabismus 2013; 50: 37-43. 5.Von Noorden GK. Amblyopia. In: Lampert R, editor. Binocular vision and ocular motility. 6th ed. St Louis: CV Mosby Company, 2002. p 246-97. 6.Bayhan HA, Aslan Bayhan S, Muhafiz E, et al. Comparison of anterior segment parameters with optical low coherence reflectometer and combined scheimpflug-placido disk topographer. Glo-Kat 2013;8:78-82. 7.Palamar M, Degirmenci C, Biler ED, et al. Evaluation of the anatomic and refractive differences in hyperopic anisometropia. Int Ophthalmol 2016; 36(6): 881-6. 8.Davidson S, Quinn GE. The impact of pediatric vision disorders in adulthood. Pediatrics 2011; 127(2): 334–9. 9.Ko?ak G, Durano?lu Y. Amblyopia and Treatment. Turk J Ophthalmol 2014; 44(3): 228–36. 10.Holmstrom M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol 1998; 82: 1265-71. 11.Weakley DR Jr. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmol 2001; 108: 163-71. 12.Abrahamsson M, Sjostrand J. Natural history of infantile anisometropia. Br J Ophthalmol 1996; 80:860-3. 13.Uretmen O, Pamukcu K, Kose S, et al. Oculometric features of hyperopia in children with accommodative refractive esotropia. Acta Ophthalmol Scand 2003; 81: 260-3. 14.Dervi?o?lu S, G?rl? V, Erda N. Factors Causing Difference in Refractive Defects Between Two Eyes in Anisometropic Amblyopia Cases Anizometropi. Balkan Medical Journal 2006; 2006(2): 65-69 15.Wang BZ, Taranath D. A comparison between the amblyopic eye and normal fellow eye ocular architecture in children with hyperopic anisometropic amblyopia. J AAPOS 2012; 16(5): 428-30. 16.Yuksel N, Yuksel E, Ozer MD. Evaluation of anterior segment parameters using the Pentacam in hyperopic anisometropic amblyopic and normal eyes. J AAPOS 2014; 18(3): 248-50. 17.Demircan S, Gokce G, Yuvaci I et al. The assessment of anterior and posterior ocular structures in hyperopic anisometropic amblyopia. Med Sci Monit 2015; 21: 1181-8. 18.Bernardo L, Isaac R, Renato A Jr. Corneal Densitometry in Keratoconus. Cornea 2014; 33 (12): 1282–1286 19.Ahmed OM, Fares U, Al-Aqaba MA, et al. Corneal Densitometry as an Indicator of Corneal Health. Ophthalmol 2012; 119(3): 501–508. 20.Weiner X, Baumeister M, Kohnen T, et al. Repeatability of lens densitometry using Scheimpflug imaging. Journal of Cataract & Refractive Surgery 2014; 40(5): 756–763. 21.Kashima K, Trus BL, Unser M, et al. Aging studies on normal lens using the Scheimpflug slit-lamp camera. Invest. Ophthalmol. Vis. Sci 1993; 34(1): 263-269. 22.Ortiz D, Ali? JL, Ruiz-Colech? J, et al. Grading nuclear cataract opacity by densitometry and objective optical analysis. Journal of Cataract & Refractive Surgery 2008; 34(8): 1345–1352. 23.Huang J, Savini G, Li J, et al Evaluation of a new optical biometry device for measurements of ocular components and its comparison with IOLMaster. Br J Ophthalmol 2014; 98: 1277-1281. 24.Satou T, Niida T, Ito M. Biometry: a tool for the detection of amblyopia risk factor in children. Graefes Arch Clin Exp Ophthalmol 2019; 257(9): 2049-2056. 25.Tekin K, Cankurtaran V, Inanc M, et al. Effect of myopic anisometropia on anterior and posterior ocular segment parameters. Int Ophthalmol. 2017; 37(2): 377-384.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors did not receive any financial support from any public or private source. Received 27.03.2020
|