J.ophthalmol.(Ukraine).2020;5:51-55.
|
http://doi.org/10.31288/oftalmolzh202055155 Received: 16 June 2020; Published on-line: 27 October 2020
OCT-measured morphological and structural parameters of the retinal ganglion cell complex in compressive optic neuropathy K. S. Iegorova1, V. V. Biloshytskyi1, M. A. Znamenska2, M. O. Guk1, A. O. Mumliev1, D. M. Tsiurupa1 1 State Institution "Romodanov Neurosurgery Institute, National Academy of Medical Sciences of Ukraine"; 2 State Institution "Institute of Pediatrics, Obstetrics and Gynecology of NAMS of Ukraine" Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE: Iegorova KS, Biloshytskyi VV, Znamenska MA, Guk MO, Mumliev AO, Tsiurupa DM. OCT-measured morphological and structural parameters of the retinal ganglion cell complex in compressive optic neuropathy. J.ophthalmol.(Ukraine).2020;5:51-5. http://doi.org/10.31288/oftalmolzh202055155 Background: Skull-base tumors (SBTs) of the middle and anterior fossae cause compressive optic neuropathy which is accompanied by decreased visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA). Optical coherence tomography (OCT) is an up-to-date non-invasive imaging modality that allows objective assessment of stereometric parameters of the optic nerve. Reduced peripapillary retinal nerve fiber layer thickness, reduced neuroretinal rim area, and reduced macular ganglion cell complex (GCC) thickness are characteristic changes in retinal morphology in compressive optic neuropathy. Purpose: To analyze the changes in OCT-measured morphological and structural parameters of the retinal GCC in patients with primary optic atrophy due to compression by SBTs. Material and Methods: This study included 57 patients (114 eyes) who received treatment for SBT and loss of visual acuity and/or visual fields at the Romodanov Neurosurgery Institute during 2017 through 2019. Patients underwent clinical and neurological, eye, otoneurological, neiroimaging and laboratory examination. Results: There was a significant difference in average GCC thickness among any subgroup (Subgroup 1, 101.69 ± 4.01 nm; Subgroup 2, 98.6 ± 2.28 nm; Subgroup 3, 90.4 ± 3.92 nm; Subgroup 4, 80.8 ± 3.72 nm; Subgroup 5, 71.86 ± 5.31 nm) and controls (113.01±3.86 nm) (p < 0.05). Although no abnormalities in visual function were found in eyes of Subgroup 1 (no chiasmal syndrome before and after treatment), these eyes exhibited GCC thinning in superior nasal and inferior nasal segments (99.24±3.21 nm and 96.21±3.18 nm, respectively), which is an early sign of compressive optic neuropathy. Conclusion: Binasal thinning of the GCC (a subclinical sign of chiasmal compression) reflects early mild damage to axons, precedes visual field defects and optic atrophy, and is an early indication for SBT removal. Keywords: skull-base tumors, chiasmal syndrome, compressive optic neuropathy, optical coherence tomography
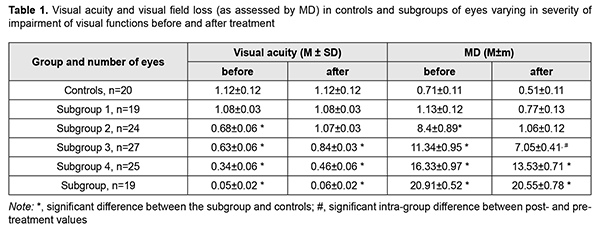
Introduction Skull-base tumors (SBTs) of the middle and anterior fossae cause compressive optic neuropathy which is accompanied by decreased visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA). Pituitary adenoma, sella turcica meningioma and supradiaphragmatic craniopharyngioma are among the most common SBTs [1, 2, 3, 4]. Loss of visual acuity and/or visual fields is an early and major symptom in the clinical picture, observed in 40-65% of patients with SBT, and may result in a temporary or permanent visual loss [5]. In patients with long-standing compression of the optic chiasm, ganglion cells may undergo axonal degeneration manifesting as retinal and optic nerve changes. Reduced peripapillary retinal nerve fiber layer (RNFL) thickness, reduced neuroretinal rim (NRR) area, and reduced macular ganglion cell complex (GCC) thickness are characteristic changes in retinal morphology. Prolonged chiasmal compression results in the development of OA in 26.7% to 72% of patients, leading to blindness in 3.5% to 25% of cases [4, 6, 7, 8]. Optical coherence tomography (OCT) is an up-to-date non-invasive imaging modality that allows objective assessment of stereometric parameters of the optic nerve [7, 9, 10, 11]. Numerous studies stressed the role of OCT in assessing RNFL thickness loss in the development of OA [12, 13, 14]. Moon and colleagues noted that visual field changes precede GCC loss. Some OCT studies were limited by small patient sample sizes, pointed that OCT had low sensitivity for diagnosing SBTs, and did not investigate segmental GCC loss [12, 15, 16]. SBT-associated loss of visual acuity and/or visual fields is an absolute indication for tumor removal. However, surgery results in improvement or restoration of visual functions not in all patients. OCT-based early detection of first signs of compressive optic neuropathy would enable establishing early indications for surgical treatment of SBTs. In addition, early surgery for small SBT would improve treatment outcomes with regard to restoration of visual functions and prevention of optic atrophy. The purpose of the study was to analyze the changes in OCT-measured morphological and structural parameters of the retinal GCC in patients with primary optic atrophy due to compression by SBTs. Material and Methods This study included 57 patients (114 eyes; 35 women and 22 men; aged 22 to 77 years; mean age, 50.2 ± 1.6 years) who received treatment for SBT and loss of visual acuity and/or visual fields at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2019. The inclusion criterion was neuroimaging (contrast MRI of the brain) evidence of primary SBT of the middle and anterior fossae. Exclusion criteria were continued tumor growth, signs of intracranial hypertension, and concomitant ocular disorders. Patients underwent clinical and neurological, eye, otoneurological (a routine otoneurological examination with assessment of cranial nerve function), neuroimaging and laboratory examination. An eye examination was conducted before and 6 and 12 months after tumor removal (chiasmal and optic nerve decompression). We believed that conducting an eye examination at these time points was reasonable because there were cases with late restoration of visual functions among patients of study groups. Examination included best-corrected visual acuity assessment, biomicroscopy, static automated and kinetic perimetry, direct and indirect ophthalmoscopy, and OCT. Static automated perimetry (SAP) was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) using the neurological 30-2 threshold test program and Neuro screening program. Aside from defect localization, the arithmetic mean of the sensitivity loss, the mean defect (MD), was used to assess visual field loss severity. OCT was conducted using a Revo NX instrument (Optopol Technology SA, Zawiercie, Poland). Optic disc excavation area, depth and volume, peripapillary RNFL thickness, NRR area, and macular GCC thickness were assessed by OCT. The GCC includes three retinal layers: the RNFL, retinal ganglion cell (RGC) layer, and inner plexiform layer (IPL). Averages GCC thickness was assessed for superior (S), superior temporal (ST), inferior temporal (IT), superior nasal (SN), and inferior nasal (IN) sectors. The main group included 57 patients (114 eyes) with SBT. These were divided into subgroups taking into account the severity of chiasmal syndrome (CS (visual acuity and visual field loss as assessed by mean deviation (MD)): Subgroup 1 of 19 eyes with no CS (visual acuity, 1.0 or better; MD, -2 dB or better) before and after treatment in the presence of compression of the optic nerve/chiasm complex by the neoplasm based on neuroimaging (MRI or CT) evidence; Subgroup 2 of 24 eyes with no CS (visual acuity, 1.0 or better; MD, -2 dB or better) after treatment; Subgroup 3 of 27 eyes with mild CS (visual acuity, 0.5 to 0.9; MD, -2 to -10 dB); Subgroup 4 of 25 eyes with moderate CS (visual acuity, 0.1 to 0.4; MD, -10 to -20 dB); and Subgroup 5 of 19 eyes with severe CS (visual acuity, <0.1 and/or MD worse than -20 dB). The control group comprised 10 individuals (20 eyes) with no concomitant ocular or neurosurgical disorder. This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. Results are presented as the mean and standard deviation (M ± SD). Student’s unpaired t-test was used to determine differences between independent groups. The level of significance p ≤ 0.05 was assumed. Spearman correlation coefficients were used to assess correlations. Results Tumor extension was classified as suprasellar in all cases, and visual impairment (reduced visual acuity and/or visual field defects) was the major manifestation of disease in most patients. Chiasmal and optic nerve decompression was achieved in all 57 patients through surgical tumor removal. Table 1 presents visual acuity and visual field loss as assessed by mean deviation (MD) for subgroups of patients.
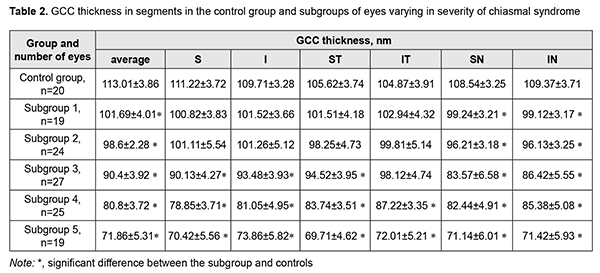
There were significant differences in visual acuity and MD between patients of Subgroups 3, 4, 5 and controls (p < 0.05), but not between patients of Subgroups 1 and 2 and controls (p > 0.05). In addition, there were no differences in visual acuity and MD between patients of Subgroup 2 and controls, although preoperatively, among eyes of this subgroup (the subgroup of eyes with no post-treatment CS), VA varied from 0.1 to 1.0 (mean value, 0.68 ± 0.06), and 16 eyes had temporal hemianopia only (mostly, relative temporal hemianopia); 3 eyes, temporal hemianopia with central scotoma; and 2 eyes, paracentral temporal scotoma. Among the subgroup of eyes with mild CS, preoperatively, VA varied from 0.1 to 1.0, and there were significant differences between preoperative and postoperative values in visual acuity (mean value, 0.63 ± 0.06, and 0.84±0.03, respectively; p < 0.05) and MD (mean value, 11.34 ± 0.95 dB, and 7.05 ± 0.41 dB, respectively; p < 0.05). In addition, preoperatively, the most common type of visual field loss was absolute temporal hemianopia (11 eyes), and postoperatively, relative temporal hemianopia (19 eyes). Among the subgroup of eyes with moderate CS, preoperatively, VA varied from 0.01 to 0.9 (mean value, 0.34 ± 0.06), and 14 eyes had temporal hemianopia with central scotoma, 10 eyes had absolute temporal hemianopia, and 1 eye had central scotoma only. Postoperatively, visual acuity improved (mean value, 0.46 ± 0.06), with no significant difference between postoperative and preoperative values (p > 0.05). In addition, the number of eyes with substantial visual field defects reduced, and visual field loss as assessed by MD improved (mean value, 13.53 ± 0.71 dB), with a significant difference between postoperative and preoperative values (p < 0.05). Among the subgroup of eyes with severe CS, postoperatively, visual acuity worsened and varied from amaurosis to 0.3 (mean value, 0.05 ± 0.02), and 10 eyes had residual visual field; and 3 eyes, absolute temporal hemianopia with central scotoma. In addition, visual field was not measurable in another 6 eyes of this subgroup. Moreover, there was no significant difference between postoperative and preoperative values of visual acuity and visual field loss as assessed by MD (p > 0.05). There was ophthalmoscopic evidence of primary descending OA in all (100%) eyes of Subgroup 5, 20 eyes (83.3%) of Subgroup 4, and 10 eyes (37%) of Subgroup 3. No fundus changes in eyes of Subgroup1 or Subgroup 2 was observed. Discussion Evidence from the literature and our previous studies of morphostructural changes in the retina and optic nerve in patients with SBTs allow us to state that retinal ganglion cell layer thinning precedes other changes in the retinal morphology and structure like RNFL thinning and reduction in NRR area. That is why the purpose of the present study was to analyze the changes in OCT-measured retinal ganglion cell layer thickness [8, 13, 15]. There was a significant difference in average GCC thickness among any subgroup (Subgroup 1, 101.69 ± 4.01 nm; Subgroup 2, 98.6 ± 2.28 nm; Subgroup 3, 90.4 ± 3.92 nm; Subgroup 4, 80.8 ± 3.72 nm; Subgroup 5, 71.86 ± 5.31 nm) and controls (113.01±3.86 nm) (p < 0.05). There were some features in the relationship between GCC thickness in segments and chiasmal syndrome severity (Table 2). In eyes of Subgroups 1 and 2, GCC thickness was reduced in segments SN (99.24±3.21 nm and 96.21 ± 3.18 nm, respectively) and IN (99.12 ± 3.17 nm and 96.13±3.25 nm, respectively) (p < 0.05), but not significantly in other segments (p > 0.05) compared to controls. In addition, in eyes of Subgroup 3, GCC thickness was reduced significantly in segments S (90.13 ± 4.27 nm), I (93.48 ± 3.93 nm), SТ (94.52 ± 3.95 nm), SN (83.57 ± 6.58 nm), and IN (86.42 ± 5.55 nm) (p < 0.05), but not significantly in segment IT (98.12 ± 4.74 nm) (p > 0.05) compared to controls. Moreover, in eyes of Subgroups 4 and 5, GCC thickness was reduced significantly in all segments compared to controls (p < 0.05).
The major sign of eyes with skull-base tumors is a gradual development of chiasmal syndrome, which is accompanied by decreased visual acuity, bitemporal visual field defects and development of primary descending OA. Our analysis of morphological and structural parameters of the retina found GCC thinning in eyes with SBTs, which is in agreement with the findings of studies by Tieger and colleagues [15], Vuong and co-authors [13], and Micieli and colleagues [14]. Although no abnormalities in visual function were found in eyes of Subgroup 1 (no CS before and after treatment), these eyes exhibited GCC thinning in superior nasal and inferior nasal segments (99.24±3.21 nm and 96.21±3.18 nm, respectively), which is an early sign of compressive optic neuropathy. Some authors [16] note that changes in the visual field precede GCC thinning, which is not in agreement with our findings. Although there was no significant difference in visual acuity, visual field loss (as assessed by MD) and GCC thickness between eyes of Subgroup 2 (no CS after treatment) and eyes of Subgroup 3 (mild CS), the treatment resulted in complete restoration of visual acuity and visual fields, but GCC thickness decreased to 98.6±2.28 nm in eyes of Subgroup 2. This suggests that loss of a portion of nerve fibers precedes irreversible visual function loss. In eyes with mild CS, there was GCC thinning in all segments except the superior temporal segment (98.12 ± 4.74 nm). The treatment of eyes with mild or moderate CS resulted in improvement in visual acuity and MD. Eyes with severe CS exhibited a reduction in GCC thickness to 71.86 ± 5.31 nm, with no improvement in visual acuity and MF after chiasmal decompression. These data indicate that the treatment of SBTs in eyes with severe CS resulted in preservation, but not in improvement in residual visual functions. Therefore, we believe that binasal thinning of the GCC (a subclinical sign of chiasmal compression) reflects early mild damage to axons, precedes visual field defects and optic atrophy, and is an early indication for SBT removal. Assessment of the OCT data may be used for predicting post-surgical improvement in or restoration of visual functions.
References 1.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015 Jul;76(3):210-9. 2.Predoi D, Badiu C, Alexandrescu D, Agarbiceanu C, Stangu C, Ogrezeanu I, et al. Assessment of compressive optic neuropathy in long standing pituitary macroadenomas. Acta Endocrinologica (Buc). 2008 Jan; 4(1):11-22. 3.Ekpene U, Ametefe M, Akoto H et al. Pattern of intracranial tumours in a tertiary hospital in Ghana. Ghana Med J. 2018 Jun;52(2):79-83. 4.Masaya-anon P, Lorpattanakasem J. Intracranial tumors affecting visual system: 5-year review in Prasat Neurological Institute. J Med Assoc Thai. 2008; 91(4):515-19. 5.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 6.Tagoe NN, Essuman VA., Fordjuor G, Akpalu G, Bankah P, Ndanu T. Neuro-ophthalmic and clinical characteristics of brain tumours in a tertiary hospital in Ghana. Ghana Med J. 2015; 49(3):181-6. 7.Frohman E, Fujimoto J, Frohman T, Calabresi P, Cutter G, Balcer L. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008 Dec;4(12):664-75. 8.Iegorova KS, Znamenska MA, Guk MO, Mumliev AO. Early signs of primary compressive optic atrophy evidenced by OCT in patients with basal brain tumors. Journal of Ophthalmology (Ukraine). 2020;1:35-9. 9.Schuman J, Hee M, Arya A, Pedut-Kloizman T, Puliafito C.A, Fujimoto J.F, Swanson EA. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995; 6:89–95. 10.Korol AR, Zadorozhnyy OS, Naumenko VO, et al. Intravitreal aflibercept for the treatment of choroidal neovascularization associated with pathologic myopia: A pilot study. Clin Ophthalmol. 2016 Nov 4;10:2223-2229. 11.Pasyechnikova NV, Naumenko VO, Korol AR, et al. Intravitreal ranibizumab for the treatment of choroidal neovascularizations associated with pathologic myopia: A prospective study. Ophthalmologica. 2016; 10: 2223–9. 12.Johansson C, Lindblom B. The role of optical coherence tomography in the detection of pituitary adenoma. Acta Ophthalmol. 2009 Nov;87(7):776-9. 13.Vuong L, Hedges T. Ganglion cell layer complex measurements in compressive optic neuropathy. Curr Opin Ophthalmol. 2017 Nov;28(6):573-8. 14.Micieli J, Newman N, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. 2019 Feb;32(1):115-123. 15.Tieger MG, Hedges TR 3rd, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, Mendoza-Santiesteban CE. Ganglion Cell Complex Loss in Chiasmal Compression by Brain Tumors. J Neuroophthalmol. 2017 Mar;37(1):7-12. 16.Moon CH, Hwang SC, Kim BT, et al. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011 Oct 31;52(11):8527-33.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|