J.ophthalmol.(Ukraine).2020;6:38-43.
|
http://doi.org/10.31288/oftalmolzh202063843 Received: 21 September 2020; Published on-line: 21 December 2020 Relationship of the ocular surface temperature with the clinical features of induced non-infectious anterior and intermediate uveitis in rabbits O. Dorokhova, O. Zborovska, Men Guanjun The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: dorochovaa@gmail.com TO CITE THIS ARTICLE: Dorokhova O E, Zborovska OV, Guanjun Meng. Relationship of the ocular surface temperature with the clinical features of induced non-infectious anterior and intermediate uveitis in rabbits. J.ophthalmol.(Ukraine).2020;6:38-43. http://doi.org/10.31288/oftalmolzh202063843 Background: It is an important challenge to develop objective methods for assessing intraocular inflammation. Purpose: To assess the relationship of the ocular surface temperature in the projection of the ciliary body with the clinical signs of induced non-infectious anterior and intermediate uveitis in rabbits. Material and Methods: Measurements of the ocular surface temperature in the projection of the ciliary body were performed in 17 Chinchilla rabbits with induced non-infectious anterior and intermediate uveitis. Results: The clinical signs of uveitis which characterize inflammatory activity were most pronounced on day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, gradually decreased thereafter and were not observed by day 19. There was a weak correlation between the ocular surface temperature in the projection of the ciliary body and weighted score of the clinical signs of anterior and intermediate uveitis (r = 0.32; р = 0.0001) for all follow-up time points. The difference in temperature between the affected eye and the intact eye correlated with the weighted score of clinical signs of experimental uveitis activity. Thus, this correlation was strong (r = 0.73; р = 0.008) on day 1, became weaker on subsequent days, and became not significant from day 8 (r = 0.4; р = 0.19). The difference in the ocular surface temperature in the projection of the ciliary body between the affected eye and the fellow eye does not clearly represent an ocular temperature response to the induced disease due to the presence of the autonomic nervous system (ANS) response in the form of an increase in the temperature of the fellow eye. Therefore, the presence of this ANS response should be taken into account in calculations, and the comparison should be conducted against reference temperature values of intact eyes, but not against the fellow eye. Keywords: uveitis, objectively assess inflammation, ocular surface temperature, thermoelectric device
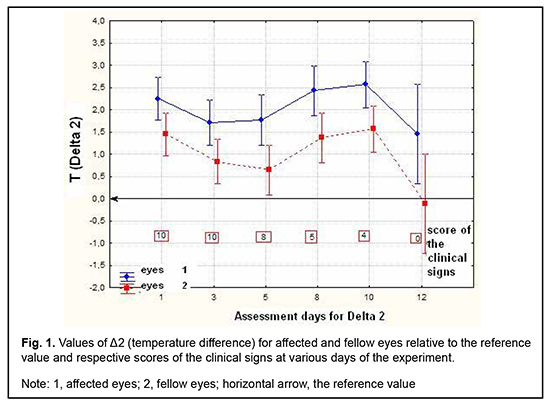
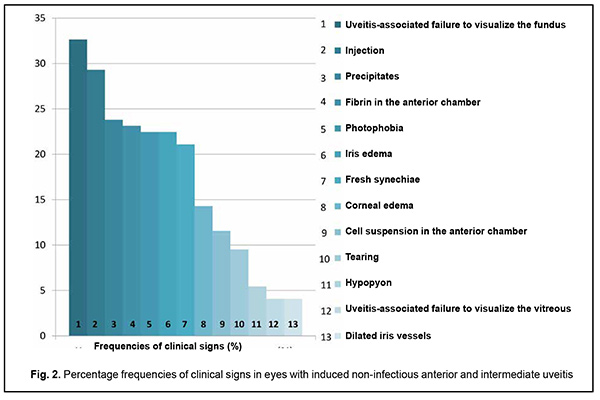
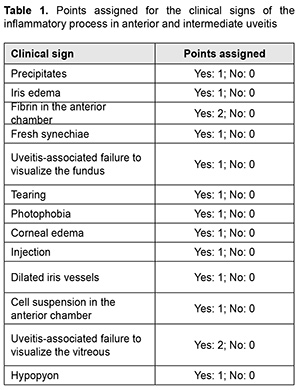
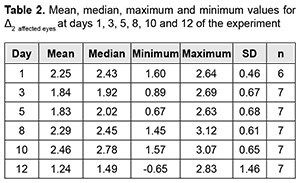
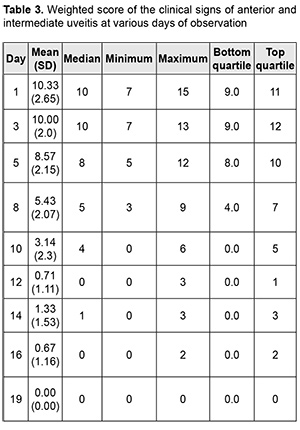
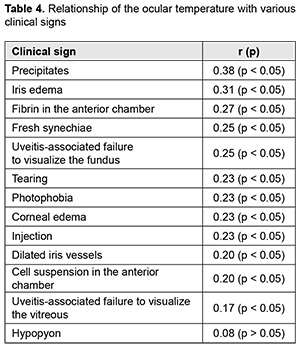
Introduction Uveitis is a group of inflammatory eye diseases that can cause catastrophic damage to ocular structures [1]. Approximately 10% to 15% of preventable blindness in Western countries is caused by uveitis and associated complications [2]. Uveitis and its complications still cause a significant burden on the healthcare system due to high prevalence of the disease [3]. The treatment paradigm for intraocular inflammation generally entails rapid initial control of inflammation, usually with systemic or local corticosteroids, along with concomitant or subsequent initiation of steroid-sparing immunomodulatory therapy for severe inflammation requiring further control. Once inflammation is controlled, systemic corticosteroids are tapered and discontinued to minimize and prevent adverse effects [4]. Making a decision on the amount and duration of immunosuppressive therapy for uveitis requires assessing the inflammatory activity of the disease. Currently, most studies use Standardization of Uveitis Nomenclature (SUN) and (National Institutes of Health (NIH) classification schemes for assessing uveitis activity [5, 6]. These classifications are based on measuring the cells and flare present in the anterior chamber and vitreous humor. It is understandable that these classifications are subjective and the results of assessments using them depend much on the examiner and his/her susceptibility to making errors. Researchers have been searching for years for simple, inexpensive and reliable methods for objective assessment of intraocular inflammation for years. Since measurement reproducibility is important, especially in clinical trials, recently, researchers have been trying to gradually change from subjective inflammation assessment methods in favor of objective inflammation assessment methods. Methods of local temperature measurements may be considered cost-effective; in addition, they are non-invasive and have no contraindications. Moreover, they are easy-to-use, informative, and safe for patients and reproducible [7]. Previously, we have found that, on day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, the temperature in ciliary body projection onto the ocular surface increased to 35.7°С (р = 0.002) for the challenged eyes and to 35.0°С (р = 0.05) for the fellow eyes compared to eyes of intact rabbits (34.1°С), which was likely to be caused by the response of the autonomic nervous system in the presence of initiation of inflammatory process. In addition, on day 5, a significant difference appeared in the ocular surface temperature between the challenged eyes and fellow eyes (36.0°С vs 34.7°С; р = 0.04). The absence of a significant difference in temperature between the affected eye and fellow eye on day 1 was likely to be caused by an increase in the temperature of the fellow eye due to the response of the autonomic nervous system. Over two weeks after intravitreal challenge for induction of the anterior and intermediate uveitis, no pathological structural change was observed in the fellow eye. This stresses that such a response of the autonomic nervous system was only functional [8, 9]. The purpose of this study was to assess the relationship of the temperature of the ocular surface in the projection of the ciliary body with the clinical signs of induced non-infectious anterior and intermediate uveitis in rabbits. Material and Methods Seventeen Chinchilla rabbits (34 eyes; weight, 2.5-3.0 kg) were included in this study. They were divided into two experimental series, series 1 (7 rabbits) and series 2 (10 rabbits). Animals were used in the experiments after being quarantined for 2 weeks. They were housed at the normal vivarium temperature of 18 до 25°С, and fed and watered conventionally. Non-infectious anterior or intermediate uveitis was induced in the right eye of each rabbit, while the left was healthy [8]. Biomicroscopy, ophthalmoscopy, measurements of ocular surface temperature in the projection of the ciliary body and inflammation scoring based on our score system were used to monitor the course of inflammation. Each variable characterizing the clinical picture was assigned one or two points based on whether the symptom is severe or not (Table 1). Such important clinical signs of a severe uveitis course as the presence of fibrin in the anterior chamber and uveitis-associated failure to visualize the vitreous were assigned a score of 2. A thermoelectric device developed within the framework of the partnership agreement between the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring ocular surface temperature in the projection of the ciliary body in animals as per the methodology described previously [10, 11]. Measurements were conducted every other day or every third day for rabbits of series 1 and every fourth to sixth day for rabbits of series 2. The follow-up period was 6 to 57 days. Two euthanasia groups were formed. Animals from the early euthanasia group were euthanized after there was no more ophthalmoscopic evidence of symptoms of active uveitis (approximately at 1-2 weeks). Animals from the late euthanasia group were euthanized after the difference in temperature between the affected eye and the fellow eye became less than or equal to 0.2 °С for consecutive measurement days. As animals were euthanized, their eyes were harvested for histomorphological studies which will be reported in the future. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Data is presented as mean ± standard deviation (SD). Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK) software. The level of significance p ≤ 0.05 was assumed. Correlation matrices were used to calculate Pearson’s and Spearman’s correlation coefficients. Results At the first stage of the study, we examined the relationship between the temperature of the ocular surface and the clinical sign score. There was a weak correlation between the ocular surface temperature in the projection of the ciliary body and weighted score of the clinical signs of anterior and intermediate uveitis (r=0.32; р=0.0001) for all follow-up time points. For convenience of subsequent calculations, we created a variable (Δ1) indicating a difference in the temperature of the ocular surface in the projection of the ciliary body between the affected eye and the fellow eye. Δ1= Т affected eye – Т fellow eye (1) Temperature differences (Δ1) for all follow-up time points were normally distributed (K-S d = 0.05, p> 0.2). Thereafter, we separated these temperature differences into four categories: category 1, those within a range from 0 to 0.5°С (mild temperature difference); category 2, those within a range from 0.5 to 1.2°С (moderate temperature difference); category 3, those within a range of at least 1.2°С (severe temperature difference); and, category 4, for a negative Δ1 (i.e., the temperature of the affected eye was lower than that of the fellow eye). At day 1, most rabbits exhibited category 2 Δ1, and there was even one rabbit exhibiting a negative Δ1. At day 1, the percentage of rabbits exhibiting category 1 Δ1, was approximately equal to that for category 2 Δ1, which indicated an increased mean temperature difference between the affected eye and the fellow eye. The highest percentage of rabbits exhibiting category 3 Δ1 (57.1%) was noted on day 5. That is, at that day, there was the greatest mean temperature difference between the affected eye and the fellow eye. The dominance of the rabbits exhibiting not the greatest temperature difference between the affected eye and the fellow eye on day 1 was likely to be caused by the response of the autonomic nervous system in the form of an increase in the temperature of the fellow eye due in the first days after uveitis onset (i.e., the response that we have reported on previously [8]). Subsequently, and until day 20, there was the dominance of rabbits exhibiting category 2 Δ1, excepting day 12 when the percentage of the rabbits exhibiting Δ1 ≥1.2°С increased again. In addition, from day 16, the days with no rabbits exhibiting a severe temperature difference (category 3 Δ1) began to appear. In the period after day 20, there was no clear tendency regarding the dominance of the rabbits exhibiting a particular temperature difference category, and the dominance of the rabbits exhibiting a mild temperature difference was changed by the dominance of the rabbits exhibiting a mild temperature difference, and vice versa. During the follow-up period, most observations had a moderate temperature difference Δ1. When assessing the percentage distribution of categories of Δ1, one should take into account that there was a gradual reduction in the number of observations due to an increase in the number of euthanized animals beginning from day 6 of the experiment, when the rabbits with clinically inactive anterior uveitis already appeared. When the experiment was approaching its end, measurements were performed only for one or two rabbits a day. In addition to day 1, from day 12, the days with some rabbits exhibiting a negative temperature difference (category 4 Δ1) began to appear. However, at these days, there was no accumulation of the percentage of rabbits exhibiting a negative temperature difference, because animals were euthanized after the difference in temperature between the affected eye and the fellow eye became less than or equal to 0.2 °С. There was no correlation between the weighted score of clinical signs and the temperature difference Δ1 (r=0.16; р > 0.05). Therefore, the difference in the ocular surface temperature in the projection of the ciliary body between the affected eye and the fellow eye (Δ1) does not clearly represent an ocular temperature response to the induced disease. In order to create an objective picture of an increase in ocular temperature, we created a variable (Δ2) indicating a difference in the temperature of the affected eye (formula 2) or the fellow eye (formula 3) and the reference temperature value. Previously, we have found a reference temperature value of 34.11°С (SD=1.421) in a study on the temperature of the ocular surface in 42 intact rabbits [11]. Δ2 affected eyes = Т affected eyes – Т intact eyes (2) Δ2 fellow eyes = Т fellow eyes – Т intact eyes (3) Over the follow-up period, there was a significant difference between the mean temperature for affected eyes and reference temperature value (p=0.0001) and between the mean temperature for fellow eyes and reference temperature value (p=0.004). Temperature differences (Δ2 affected eyes) were normally distributed (K-S d = 0.07, p> 0.2). The median value for Δ2 affected eyes was 1.16, (minimum value, -2.0; maximum value, 3.12; bottom quartile, 1.16; top quartile, 1.92). Calculation of Δ2 affected eyes and weighted score of clinical signs was performed only for the rabbits of the experimental series 1 (7 rabbits) because measurements were performed more often for these rabbits (every other day or every third day) than for rabbits of series 2 (every fourth to sixth day). In day 1 to day 10 of the experiment, there was an apparently increased temperature response of the affected eyes relatively the reference temperature value (Δ2). The largest temperature difference between the affected eyes and intact eyes was noted at day 8 and day 10, whereas thereafter, Δ2 decreased significantly (Table 2). The mean weighted score of clinical signs was largest on day 1 of the experiment, and gradually decreased thereafter. From day 10, a minimum value of 0 began to appear, with bottom quartile and median values of 0. By day 19, the median, minimum, maximum, top quartile and bottom quartile values for the weighted score of clinical signs were equal to zero (Table 3). The temperature difference Δ2 (but not Δ1, the difference in temperature between the affected eye and the fellow eye) was mildly and significantly correlated with the weighted score of clinical signs (r=0.3; р=0.0001). Changes in the temperature difference Δ2 and the weighted score of clinical signs over time are presented in Fig. 1. It is seen that variations in Δ2 for affected and fellow eyes are correlated due to the autonomic nervous system response in the form of an increase in temperature of the fellow eye. In addition, not a variation, but a gradual decrease in the weighted score of clinical signs over time was observed. At day 12, there was already no difference between the temperature for the fellow eye and the reference temperature value, and the median for the weighted score of clinical signs was 0 (Fig. 1). At day 1, Δ2 affected eyes was strongly correlated with the weighted score of clinical signs (r=0.73; р=0.008). Subsequently, this correlation became weaker (days 3, r=0.54, p=0.05; day 5, r=0.68, р=0.02). This correlation became not significant from day 8 (r=0.4; р=0.19) and absent at day 12 (r=-0.09; р=0.7). Frequencies for clinical signs in 147 affected eyes are shown in Fig. 2. Statistically significant but mild Spearman correlations were detected between the temperature for the affected eye and each clinical sign with the exception of hypopyon. Ranked correlation coefficients are shown in Table 4. The ocular surface temperature in the projection of the ciliary body was found to be affected by all clinical signs of uveitis with the exception of hypopyon and uveitis-associated failure to visualize the ocular fundus. Discussion In an article on clinical and functional evaluation of ocular inflammatory disease using the model of experimental autoimmune uveitis, Chen and Caspi [12] noted that the methods for systematic evaluation of disease using noninvasive clinical assessments should be used to facilitate longitudinal follow-up. Our methodology conforms to the proposed requirements for assessing experimental uveitis. Previous studies have attempted to establish relationships between clinical signs of uveitis and changes in local temperatures of the eye. In a study on corneal thermal patterns in anterior uveitis, Mapstone [13] used a bolometer for measurements of corneal, limbal and lower lid skin temperatures. From these measurements the temperature differences between the side of the inflamed and normal eye were calculated (∆t). Mapstone found that unilateral acute anterior uveitis caused increases in corneal temperature and periorbital skin temperature at the side of the inflamed eye, and noted a highly significant positive linear correlation between the maximum corneal temperature increase recorded during an acute inflammation and the duration of ciliary injection. As opposed to Mapstone, we found it incorrect to compare the side of the inflamed and healthy eye due to ANS response of the fellow eye. We believe it more correct to compare the temperature of the ocular surface with reference values (Δ2). Our calculations were found to be sensitive enough to establish correlations of the temperature of the ocular surface with various clinical signs of uveitis. Statistically significant but mild Spearman correlations were detected between the temperature for the affected eye and each clinical sign with the exception of hypopyon. Others have reported previously on associations of clinical signs of ocular disorders with changes in ocular temperature. A study in rabbits [14] demonstrated that the ocular surface temperature indicated the stage of corneal wound healing, and correlated with the total response. Therefore, this study found that the clinical signs of uveitis which characterize inflammatory activity were most pronounced on day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, gradually decreased thereafter and were not observed by day 19. In addition, there was a weak correlation between the temperature of the ocular surface in the projection of the ciliary body and weighted score of clinical signs (r=0.32; р=0.0001) for all follow-up time points. Moreover, significant (р < 0.05) correlations were detected between the ocular surface temperature for the affected eye and each clinical sign with the exception of hypopyon. Finally, the difference in temperature between the affected eye and the intact eye correlated with the weighted score of clinical signs of experimental uveitis activity. Thus, this correlation was strong (r=0.73; р=0.008) on day 1, became weaker on subsequent days, and became not significant from day 8 (r=0.4; р=0.19).
References 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14 (5-6): 303-8. 2.Dick AD, Tundia N, Sorg R, et al. Risk of Ocular Complications in Patients with Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis. Ophthalmology. 2016(3);123:655–62. 3.Chu DS, Johnson SJ, Mallya UG, et al. Healthcare costs and utilization for privately insured patients treated for non-infectious uveitis in the USA. J Ophthalmic Inflamm Infect. 2013;3:64. 4.Kim JS, Knickelbei JE, Nussenblatt RB, Sen HN . Int Ophthalmol Clin. Summer 2015;55(3):79-110. 5.Bloch-Michel E, Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group: recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987 Feb 15;103(2):234–5. 6.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-16. 7.Zhuravliov AS, Karchynskyi OO. [Infrared thermography-based diagnosis of acute paranasal sinusitis]. Rynologiia. 2017;2:10-20. Ukrainian. 8.Dorokhova O, Zborovska O, Meng Guanjun. Changes in temperature of the ocular surface in the projection of the ciliary body in the early stages of induced non-infectious uveitis in rabbits. J Ophthalmol (Ukraine). 2020;3(494):47-52. 9.Dorokhova O, Zborovska O, Meng Guanjun. Histomorphology of the healthy fellow eye in the rabbit with unilaterally induced non-infectious anterior and intermediate uveitis. J Ophthalmol (Ukraine). 2020; 4(495):45-9. 10.Anatychuk LI, Pasyechnikova NV, Zadorozhnyy OS, Nazaretian RE, Myrnenko VV, Kobylyanskyi RR, Gavryliuk NV. Original device and approaches to the study of temperature distribution in various eye segments (experimental study). J Ophthalmol (Ukraine). 2015;6:50-3. 11.Dorokhova O, Zborovska O, Meng Guanjun. Temperature of the ocular surface in the projection of the ciliary body in rabbits. J Ophthalmol (Ukraine). 2020;2 (493):65-69. 12.Chen J, Caspi R. Clinical and Functional Evaluation of Ocular Inflammatory Disease Using the Model of Experimental Autoimmune Uveitis. Immunological Tolerance. 2019. Methods Mol Biol. 2019;1899:211-27. 13.Mapstone R. Corneal Thermal Patterns in Anterior Uveitis. Br J Ophthalmol. 1968; 52 (12):917-21. 14.Coles WH, Pandya RK, Anbar M, et al. Ocular surface temperature (ocular thermography) as a predictor of corneal wound healing. Invest Ophthalmol Vis Sci. 1988;29:313. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|