J.ophthalmol.(Ukraine).2021;2:10-15.
|
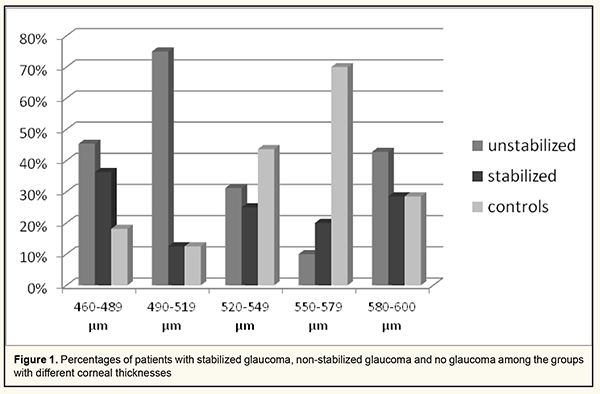
http://doi.org/10.31288/oftalmolzh202121015 Received: 15 December 2020; Published on-line: 19 April 2021 Relationships between target intraocular pressure and central corneal thickness in stabilized and non-stabilized glaucoma patients Dmitriev S. K., Peretiagin O. A., Lazar Yu.M., Tatarina Yu.A. SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: tatarina.j.a@gmail.com TO CITE THIS ARTICLE: Dmitriev SK, Peretiagin OA, Lazar YuM, Tatarina YuA. Relationships between target intraocular pressure and central corneal thickness in stabilized and non-stabilized glaucoma patients. J.ophthalmol.(Ukraine).2021;2:10-15.http://doi.org/10.31288/oftalmolzh202121015 Background: Until now, no direct relationship has been established between the target pressure and particular corneal thickness for measurements with different types of tonometers. Purpose: To establish the dependence of target intraocular pressure (IOP) on corneal thickness for Maklakoff applanation tonometry, Pascal dynamic contour tonometry (DCT) and ICare rebound tonometry. Material and Methods: Fifty-two patients (52 eyes; mean age, 71.2 ± 7.7 years) with preoperative cataract underwent an eye examination. These included 13 eyes with cataract associated with stabilized primary open-angle glaucoma (POAG), 20 eyes with cataract associated with non-stabilized POAG, and 19 control eyes with cataract and no history of or present glaucoma. Patients underwent a comprehensive eye examination, Maklakoff applanation tonometry, Pascal dynamic contour tonometry (DCT) and ICare rebound tonometry with IC 200. Results: Group 1 (corneal thickness, 460-489 μm; mean corneal thickness, 479 ± 7.2 μm) patients with stabilized glaucoma had the highest mean values for IOP obtained by any of the three methods at which no glaucoma progression was observed during the most recent 6 months. In group 1 patients with stabilized glaucoma, mean IOP was 16.9 ± 1.2 mmHg for Pascal DCT, 16.2 ± 1.4 mmHg for ICare tonometry, and 20 ± 0.8 mmHg for Maklakoff applanation tonometry. There was a significant difference (р < 0.05) in Maklakoff applanation tonometry IOP, but not in Pascal DCT or ICare tonometry, between group 1 patients with non-stabilized glaucoma (23.8 ± 2.0 mmHg) and those with no glaucoma (16.5 ± 0.7 mmHg). The highest IOP readings were observed in patients of group 5 (corneal thickness, 580–600 μm; mean corneal thickness, 593 ± 4.3 μm) with non-stabilized glaucoma: mean IOP was 28.1 ± 15.0 mmHg for Pascal DCT, 33.2 ± 21.1 mmHg for ICare tonometry, and 25.3 ± 7.5 mmHg for Maklakoff applanation tonometry. Conclusion: Maklakoff tonometry measurements are more sensitive than Pascal DCT or ICare rebound tonometry measurements in patients with thinner corneas. Sensitivity of the tonometry methods under study decreased with increases in corneal thickness and IOP. This should be taking in account while setting a target IOP for glaucoma patients. Among the groups with different corneal thicknesses (groups 1 to 5), group 2 (corneal thickness, 490 – 519 μm) had the highest percentage of patients with non-stabilized glaucoma (75%), followed by group 1 (corneal thickness, 460 – 489 μm; 45.4%). Keywords: primary open-angle glaucoma, target intraocular pressure, corneal thickness
Introduction Glaucoma is one of the most common causes of blindness in the world and one of the most discussable topics among ophthalmologists. The European Glaucoma Society guidelines (2014) define target IOP as the upper limit of the IOP estimated to be compatible with a rate of progression sufficiently slow to maintain vision-related quality of life in the expected lifetime of the patient. According to the definition of the World Glaucoma Association 7th Consensus Meeting on the Medical Treatment of Glaucoma, the target IOP is the IOP range at which the clinician judges that progressive disease is unlikely to affect the patient’s quality of life [1]. The most relevant recommendations for achieving the target IOP are as follows: ●The lower the pretreatment pressure, the lower the target pressure must be to protect the eye from further damage. ●The more advanced the damage, the lower the initial target IOP. ●The higher the rate of progression, the lower must be the target IOP. ●The target IOP for young patients should be lower than for elderly patients. Most ophthalmologists currently adhere to these recommendations. Although these recommendations are certainly relevant to current practice, their disadvantage is that they do not specify particular values and ranges for target IOP. Studies have demonstrated that the target IOP increases with blood pressure [2]. More recent studies have demonstrated that, in the presence of arterial hypotension in patients with early glaucoma, the IOP should be reduced to a low normal range of 9-14 mmHg [3,4,5]. The Advanced Glaucoma Intervention Study (AGIS) found that eyes with IOPs of <18 mmHg at all visits during the first 6 years of follow-up were least likely to show worsening [6]. The Russian National Guidelines for Glaucoma management (2015) recommends a reduction in IOP by 20%, 30% or 40% from pre-treatment level [7]. Volkov [8] believes that, in early glaucoma, the true IOP (P0) should be 18-21 mmHg, whereas in far-advanced glaucoma, the tonometric IOP (Pt) should be 12-17 mmHg. This is close to the values reported by Nesterov [9] for early glaucoma (a P0 of 19 mmHg) and far-advanced glaucoma (a Pt of 12-17 mmHg). The corneal thickness is another factor significantly influencing the target IOP. The true IOP recommended for eyes with thin cornea (<520 μm) is approximately 15.0 mmHg, whereas for eyes with moderately thin cornea and thick cornea, 17.0 mmHg and 22.0 mmHg, respectively [10,11]. Imbalance between biomechanical characteristics of the sclera and cornea may be a factor of primary open-angle glaucoma (POAG) progression in patients with normalized IOP, necessitating hypotensive therapy or glaucoma surgery for stabilization of glaucoma process [12]. No direct relationship has been established between the target pressure and particular corneal thickness for measurements with different types of tonometers. However, it is known how the corneal thickness influences Goldmann or Maklakoff IOP readings. Goldmann tonometry is the gold standard to measure IOP, but Goldmann IOP readings largely depend on corneal thickness. This is because at the time of developing the Godmann tonometer, it was believed that all human eyes were similar in volume and corneal dimensions and mechanical parameters [13]. It has been demonstrated that thinner corneas have higher true IOP readings obtained by Godmann tonometry. Tables have been developed for calculating true Goldmann IOP adjusted by corneal thickness [14]. Maklakoff tonometry readings depend on the corneal thickness, biomechanical characteristics of the sclera and in some way on the force applied by the examiner during measurements. Maklakoff tonometry is currently the most common tonometry technique in Ukraine, especially under polyclinical environment, due to easiness of application and low cost. The thicker the cornea, the higher the Maklakoff tonometry readings. There was a statistically significant relationship between the corneal thickness and IOP readings obtained with a Maklakoff tonometer with a load of 5 g and that with a load of 10 g, although the difference was less significant for the latter [15]. Pascal dynamic contour tonometry (DCT) readings are least dependent on corneal thickness. DCT employs a contoured tip which features a concave surface to conform the cornea to its inner curvature, prevents corneal deformation and reduces the influence of corneal thickness and rigidity on IOP measurement readings. The so-called contour-matched tonometer tip has a concave surface that allows the cornea to assume the shape that it naturally assumes when pressure on both sides of the cornea is equal and distortion of the cornea is minimal. The mean difference in IOP readings between Goldmann tonometer and DCT increased with increased central corneal thickness [16,17,18]. Icare IC 200 is a tonometer used in ICare® tonometry (ICT), a new method of IOP measurement based on the principle of rebound tonometry. The disposable probe bounces off the cornea and the impedance that is detected is used to calculate the IOP. As several studies found good correlation between ICT and Goldmann applanation tonometry [19, 20], the ICare rebound tonometer is increasingly used in Ukraine. The purpose of this study was to establish the dependence of target IOP on corneal thickness for Maklakoff applanation tonometry, DCT and ICare rebound tonometry. Material and Methods Fifty-two patients (52 eyes; age, 46 to 82 years; mean age, 71.2 ± 7.7 years; 30 (57.6%) males and 22 (42.3%) females) with preoperative cataract underwent an eye examination. These included 13 eyes with cataract associated with stabilized POAG, 20 eyes with cataract associated with non-stabilized POAG, and 19 control eyes with cataract and no history of or present glaucoma. Patients with glaucoma had stage 1, 2 or 3 POAG. Patients with other ocular disorders were excluded. Of the total study patients, 38.4% had cataract associated with stabilized POAG; 25%, cataract associated with non-stabilized POAG; and 36.5%, cataract with no glaucoma. Particularly, of the total study patients, 33 (64.4%) had cataract associated with stabilized or non-stabilized POAG. Patients underwent a comprehensive eye examination, which included visual acuity assessment with Sivtsev-Golovin charts, autokeratometry and autorefractometry, perimetry, slit-lamp examination, ophthalmoscopy, pachymetry, keratotopography, optic A-scanning, tonography, blood pressure measurements, Maklakoff applanation tonometry, Pascal DCT and ICare rebound tonometry with IC 200. As many foreign studies have reported on a strong association between Goldmann tonometry values and the central corneal thickness resulting in a large GAP IOP measurement error, GAP was not used in this study. Assessment of morphometric parameters by HFA II Central 30-2 Threshold Test and SOCT Copernicus+ was used to monitor glaucoma progression. Glaucoma was considered stabilized if there was no glaucomatous optic neuropathy progression during 6 month of follow-up. Maklakoff applanation tonometry was used to obtain tonometric IOP values, whereas Pascal DCT and ICare rebound tonometry with IC 200 were used to obtain true IOP values. The methods recommended for medical studies were used for statistical analysis. The data obtained were coded using a numerical, ordinal or nominal scale, and put into the developed form. Statistica 6.0 and Microsoft Excel software were used for database formation, statistical and graphical data analysis. Means and standard error of means were calculated for numerical data. Student t-test was used to assess differences in normally distributed variables. Statistical significance was set as P < 0.05. The study adhered to the tenets of the Declaration of Helsinki. Results Study eyes were divided into 5 groups based on corneal thickness (with each group including both eyes with cataract associated with stabilized or non-stabilized glaucoma and eyes with cataract only): group 1, 460 to 489 μm; group 2, 490 to 519 μm; group 3, 520 to 549 μm; group 4, 550 to 579 μm; group 5, 580 to 600 μm. Group 1 included 11 eyes (mean corneal thickness, 479.1±7.2 μm). Among patients of this group, true IOP varied from 13.8 to 39.0 mmHg for Pascal DCT, from 10.2 to 38.1 mmHg for ICare rebound tonometry and from 16 to 26 mmHg for Maklakoff applanation tonometry. Of the patients of this group, 2 (18.1%) had no glaucoma, 5 (45.4 %) had non-stabilized glaucoma, and 4 (36.3 %) had stabilized glaucoma. In group 1 patients with stabilized glaucoma, mean IOP was 16.9 ± 1.2 mmHg for Pascal DCT, 16.2 ± 1.4 mmHg for ICare tonometry, and 20 ± 0.8 mmHg for Maklakoff applanation tonometry. In group 1 patients with non-stabilized glaucoma, mean IOP was 25.2 ± 6.6 mmHg for Pascal DCT, 26.0 ± 7.5 mmHg for ICare tonometry, and 23.8 ± 2.0 mmHg for Maklakoff applanation tonometry. In group 1 patients with no glaucoma, mean IOP was 15.5 ± 2.4 mmHg for Pascal DCT, 11.7 ± 2.1 mmHg for ICare tonometry, and 16.5 ± 0.7 mmHg for Maklakoff applanation tonometry. Group 2 included 8 eyes (mean corneal thickness, 509.1±7.1 μm). Among patients of this group, true IOP varied from 14.0 to 29.7 mmHg for Pascal DCT, from 12.0 to 28.3 mmHg for ICare rebound tonometry and from 14 to 23 mmHg for Maklakoff applanation tonometry. Of the patients of this group, 1 (12.5%) had no glaucoma, 6 (75 %) had non-stabilized glaucoma, and 1 (12.5 %) had stabilized glaucoma. In group 2 patients with stabilized glaucoma, mean IOP was 14.0 mmHg for Pascal DCT, 12.0 mmHg for ICare tonometry, and 14.0 mmHg for Maklakoff applanation tonometry. In group 2 patients with non-stabilized glaucoma, mean IOP was 24.8 ± 4.2 mmHg for Pascal DCT, 24.8 ± 3.6 mmHg for ICare tonometry, and 21.5 ± 1.2 mmHg for Maklakoff applanation tonometry. In group 2 patients with no glaucoma, mean IOP was 14.6 mmHg for Pascal DCT, 13.2 mmHg for ICare tonometry, and 15.0 mmHg for Maklakoff applanation tonometry. Group 3 included 16 eyes (mean corneal thickness, 535.8 ± 9.5 μm). Among patients of this group, true IOP varied from 12.9 to 36.0 mmHg for Pascal DCT and from 9.1 to 52.1 mmHg for ICare rebound tonometry, and tonometric IOP varied from 16 to 38 mmHg for Maklakoff applanation tonometry. Of the patients of this group, 7 (43.7%) had no glaucoma, 5 (31.2 %) had non-stabilized glaucoma, and 4 (25 %) had stabilized glaucoma. In group 3 patients with stabilized glaucoma, mean IOP was 16.3 ± 1.6 mmHg for Pascal DCT, 13.4 ± 2.7 mmHg for ICare tonometry, and 19.0 ± 1.6 mmHg for Maklakoff applanation tonometry. In group 3 patients with non-stabilized glaucoma, mean IOP was 26.2 ± 7.8 mmHg for Pascal DCT, 30.2 ± 13.9 mmHg for ICare tonometry, and 26.4 ± 7.8 mmHg for Maklakoff applanation tonometry. In group 3 patients with no glaucoma, mean IOP was 15.1 ± 2.0 mmHg for Pascal DCT, 14.5 ± 3.2 mmHg for ICare tonometry, and 18.2 ± 1.8 mmHg for Maklakoff applanation tonometry. Group 4 included 10 eyes (mean corneal thickness, 560 ± 6.5 μm). Among patients of this group, true IOP varied from 10.2 to 20.1 mmHg for Pascal DCT and from 9.8 to 16.5 mmHg for ICare rebound tonometry, and tonometric IOP varied from 14.0 to 23.0 mmHg for Maklakoff applanation tonometry. Of the patients of this group, 7 (70%) had no glaucoma, 1 (10 %) had non-stabilized glaucoma, and 4 (20 %) had stabilized glaucoma. In group 4 patients with stabilized glaucoma, mean IOP was 15.5 ± 0.7 mmHg for Pascal DCT, 15.4 ± 1.3 mmHg for ICare tonometry, and 17.0 ± 1.4 mmHg for Maklakoff applanation tonometry. In group 4 patients with non-stabilized glaucoma, mean IOP was 20.1 mmHg for Pascal DCT, 16.5 mmHg for ICare tonometry, and 23.0 mmHg for Maklakoff applanation tonometry. In group 4 patients with no glaucoma, mean IOP was 13.7 ± 2.1 mmHg for Pascal DCT, 11.6 ± 1.7 mmHg for ICare tonometry, and 17.2 ± 3.0 mmHg for Maklakoff applanation tonometry. Group 5 included 7 eyes (mean corneal thickness, 593 ± 4.3 μm). Among patients of this group, true IOP varied from 13.1 to 45.3 mmHg for Pascal DCT and from 5.7 to 57.5 mmHg for ICare rebound tonometry, and tonometric IOP varied from 15.0 to 34.0 mmHg for Maklakoff applanation tonometry. Of the patients of this group, 2 (28.5%) had no glaucoma, 3 (42.8 %) had non-stabilized glaucoma, and 2 (28.5 %) had stabilized glaucoma. In group 5 patients with stabilized glaucoma, mean IOP was 14.6 ± 1.0 mmHg for Pascal DCT, 16.1 ± 1.4 mmHg for ICare tonometry, and 16.0 ± 1.4 mmHg for Maklakoff applanation tonometry. In group 5 patients with non-stabilized glaucoma, mean IOP was 28.1 ± 15.0 mmHg for Pascal DCT, 33.2 ± 21.1 mmHg for ICare tonometry, and 25.3 ± 7.5 mmHg for Maklakoff applanation tonometry. In group 5 patients with no glaucoma, mean IOP was 13.7 ± 0.9 mmHg for Pascal DCT, 11.2 ± 7.7 mmHg for ICare tonometry, and 17.0 ± 1.4 mmHg for Maklakoff applanation tonometry.
Discussion Determining a target IOP and establishing and using a link between the target IOP and an objective characteristic like the corneal thickness, corneoscleral rigidity or axial length is a major task in glaucoma management. In the current study, we determined mean values for IOP readings obtained by different tonometers at different central corneal thicknesses. Group 1 (corneal thickness, 460-489 μm; mean corneal thickness, 479 ± 7.2 μm) patients with stabilized glaucoma had the highest mean values for IOP obtained by any of the three methods at which no glaucoma progression was observed during the most recent 6 months. In these patients, mean IOP was 16.9 ± 1.2 mmHg for Pascal DCT, 16.2 ± 1.4 mmHg for ICare tonometry, and 20 ± 0.8 mmHg for Maklakoff applanation tonometry. That is, for patients with the thinnest cornea (corneal thickness, 460-489 μm), these Pascal DCT, ICare tonometry, Maklakoff applanation tonometry IOP readings may be considered target IOP values. There was a significant difference (р < 0.05) in Maklakoff applanation tonometry IOP, but not in Pascal DCT or ICare tonometry, between group 1 patients with non-stabilized glaucoma (23.8 ± 2.0 mmHg) and those with no glaucoma (16.5 ± 0.7 mmHg). In addition, there was no significant difference (р > 0.05) in Maklakoff applanation tonometry IOP, Pascal DCT or ICare tonometry between patients of other groups with glaucoma and those with no glaucoma. This may indicate that Maklakoff tonometry measurements are more sensitive than Pascal DCT or ICare tonometry measurements in patients with thinner corneas. This is likely due to the fact that tonometers measuring true IOP may overestimate IOP if IOP ≥ 20 mmHg. The results obtained are understandable given that, in group 1 patients with non-stabilized glaucoma, mean IOP was 25.2 ± 6.6 mmHg for Pascal DCT, 26.0 ± 7.5 mmHg for ICare tonometry, and 23.8 ± 2.0 mmHg for Maklakoff applanation tonometry. The manufacturer of Icare ІС 200 estimates the measurement error to be no more than 1.2 mmHg for IOP ≤ 20 mmHg and no more than 2.2 mmHg for IOP ≥ 20 mmHg. This is in agreement with findings of a study by Volkova and colleagues [21], who reported a significant magnitude of difference in IOP measurements in the high normal IOP range for Icare tonometry, and more accurate IOP measurements in this range for Maklakoff applanation tonometry. In the current study, the highest IOP readings were observed in patients of group 5 (corneal thickness, 580–600 μm; mean corneal thickness, 593 ± 4.3 μm) with non-stabilized glaucoma: mean IOP was 28.1 ± 15.0 mmHg for Pascal DCT, 33.2 ± 21.1 mmHg for ICare tonometry, and 25.3 ± 7.5 mmHg for Maklakoff applanation tonometry. However, the greatest standard deviations of IOP measurements were also noted in patients of this group. These findings confirm those of others that eyes with thicker corneas have higher IOP readings [22]. However, given that the highest IOP readings and the greatest standard deviations of IOP measurements were noted in non-stabilized glaucoma patients from the group with the thickest corneas, the sensitivity of true IOP measurements (for Pascal DCT, and ICare tonometry) and tonometric IOP measurements (for Maklakoff applanation tonometry) decreases with increases in IOP and corneal thickness. This should be taking in account while setting a target IOP for glaucoma patients. Among the groups with different corneal thicknesses (groups 1 to 5), group 2 (corneal thickness, 490 – 519 μm) had the highest percentage of patients with non-stabilized glaucoma (75%), followed by group 1 (corneal thickness, 460 – 489 μm; 45.4%) (Figuree 1). This is in agreement with findings of others that thin cornea is a risk factor for glaucoma progression, because IOP is underestimated in eyes with thinner corneas, leading to overdiagnosis of glaucoma [22, 23]. Therefore, the corneal thickness influences IOP measurements, and sensitivity of the tonometry methods under study decreased with increases in corneal thickness and IOP. Maklakoff applanation tonometry had the highest accuracy compared to Pascal DCT and ICare tonometry, but only for patients with thin cornea (460 – 486 μm). Thin cornea is a risk factor for glaucoma progression, and IOP is underestimated in eyes with thinner corneas, leading to overdiagnosis of glaucoma. These should be taken in account while selecting a target IOP for glaucoma patients. References 1.Weinreb RN, Liebmann J, World Glaucoma Association. Medical Treatment of Glaucoma: The 7th Consensus Report of the World Glaucoma Association. Kugler Publications: Amsterdam; 2010. 2.Boriskina LN. [Campimetric method for measuring the individually tolerated intraocular pressure in primary open-angle glaucoma]. In: [Aktualnyie voprosy eksperimentalnoi i klinicheskoi meditsiny]. Volgograd: VGMI; 1981. p.146-7. Russian. 3.Fokin VP, Balalin SV. [Determining target intraocular pressure in patients with primary open-angle glaucoma]. Glaucoma. 2007;4:16-20. Russian. 4.Fokin VP, Balalin SV. [Study of optic nerve intolerance to compressive ocular hypertension by computer-aided suprathreshold static perimetry in patients with glaucoma, pseudoglaucoma and ocular hypertension]. Glaucoma. 2008;2:3-8. Russian. 5.Fokin VP, Balalin SV. [Modern organizational and medical technologies in the diagnosis and treatment of primary glaucoma]. Oftal'mokhirurgiia. 2011;(2):43-9. Russian. 6.The Advanced Glaucoma Intervention Study (AGIS): 7.The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophtalmol. 2000;4:429-40. 7.[National guidelines for glaucoma for medical practitioners]. Egorov EA, Astakhov YS, Yerichev VP, editors. 3rd ed. Moscow: GEOTAR-Media; 2015. Russian. 8.Volkov VV. [Open-angle glaucoma]. Moscow: Meditsinskoie informatsionnoie agenstvo; 2008. Russian. 9.Nesterov AP. [Glaucoma]. Moscow: Meditsinskoie informatsionnoie agenstvo; 2008. Russian. 10.Egorov EA, Vasina MV. [Influence of corneal thickness on intraocular pressure among different groups of patients]. Klinicheskaia oftalmologiia. 2006;7(1):16-9. Russian. 11.Egorov EA, Vasina MV. [Intraocular pressure and corneal thickness]. Glaucoma. 2006;(2):34-6. Russian. 12.Iomdina EN, Kiseleva OA, Moiseeva IN, Shtein AA, Bessmertnyi AM, etc. [Biomechanical criteria for estimating the risk of primary open-angle glaucoma progression]. Klinicheskaia meditsina. 2016;8(4):59-63. Russian. 13.Iomdina EN, Bauer SM, Kotlyar KE. Neroev VV, editor. [Biomechanics of the eye: theoretical aspects and clinical applications]. Moscow: Real Time; 2015. Russian. 14.Kohlhaas M, Boehm AG, Spoerl E, Pursten A, Grein HJ, Pillunat LE. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006; 124(4): 471-6. 15.Alekseev VV. [Assessing the influence of corneal parameters on tonometry results in the healthy population]. Klinicheskaia oftalmologiia. 2008;(4):128. Russian. 16.Doyle A, Lachkar Y. Comparison of dynamic contour tonometry with Goldman applanation tonometry over a wide range of central corneal thickness. J Glaucoma. 2005 Aug;14(4):288-92. 17.Kaufmann C, Bachmann LM, Thiel MA. Comparison of dynamic contour tonometry with Goldmann applanation tonometry. Invest Ophthalmol Vis Sci. 2004 Sep;45(9):3118-21. 18.Kostova S, Angelov B, Petkova N. [A comparative study of intraocular pressure in primary open angle glaucoma, measured by PASCAL dynamic contour tonometer, GOLDMANN applanation tonometry and Maklakov applanation tonometry]. Klinicheskaya oftal’mologiya. 2009;10(4):123-–125. 19.Pakrou N, Gray T, Mills R, Landers J, Craig J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma. Jan-Feb 2008;17(1):43-7. 20.Fernandes P, Diaz-Rey JA, Gonzalez-Meijome JM, et al. Comparison of ICare rebound tonometer with Goldmann applanation tonometer in a normal population. Ophthal Physiol Opt. 2005;25:436–40. 21.Volkova NV, Yurieva TN, Shvets LV, Mikhalevich IM. [Correlation and correction factors for different types of tonometry. Report 1]. Natsionalnyi zhurnal glaukoma. 2015;14(3):11-8. Russian. 22.Vasina MV. [Influence of central corneal thickness on intraocular pressure and the course of glaucoma]. Thesis for the Degree of Cand Sc (Med). Moscow: Russian State Medical University; 2010. Russian.
23.Alekseev VV, Litvin IB. [Influence of corneal thickness on intraocular pressure and prognosis of glaucoma]. Klinicheskaia oftalmologiia. 2008; 9(4):23-4. Russian. Conflict of interest statement: None declared.
|