J.ophthalmol.(Ukraine).2021;2:31-35.
|
http://doi.org/10.31288/oftalmolzh202123134 Received: 02 October 2020; Published on-line: 19 April 2021 Features of dry eye manifestations in patients with different durations of diabetes mellitus I. F. Nabieva1, F. A. Khaidarova1, F. A. Bakhritdinova2, O. I. Oripov2 1 Republican Specialized Scientific and Practical Medical Center of Endocrinology; Tashkent (Uzbekistan) 1 Tashkent Medical Academy; Tashkent (Uzbekistan) E-mail: okil.oripov@mail.ru TO CITE THIS ARTICLE: Nabieva IF, Khaidarova FA, Bakhritdinova FA, Oripov OI. Features of dry eye manifestations in patients with different durations of diabetes mellitus. J.ophthalmol.(Ukraine).2021;2:31-35.http://doi.org/10.31288/oftalmolzh2021231354 Background: The risk of the development of proliferative diabetic retinopathy as well as abnormalities in the structure and/or function of the lacrimal functional unit significantly increases with an increase in diabetes mellitus duration, potentially leading to the development of dry eye. Purpose: To assess the state of the lacrimal functional unit in diabetic patients with dry eye and different durations of diabetes. Material and Methods: One hundred and twenty patients with both type 2 diabetes mellitus and dry eye disease were included in the study. Hemoglobin A1c levels and clinical manifestations of diabetic polyneuropathy and dry eye disease were comprehensively assessed. Results: We found that diabetes duration plays a dominant role in the nature of the course of dry eye disease in patients with type 2 diabetes mellitus. The main features of manifestations of dry eye disease in patients with diabetes mellitus are the predominance of the subjective symptoms in those with shorter diabetes duration and the absence of complaints of eye dryness as well as the presence of objective structural changes in the lacrimal functional unit in those with longer diabetes duration. Conclusion: Patients with long duration of diabetes mellitus have a latent course of dry eye disease and relatively severe structural abnormalities in the lacrimal functional unit. Keywords: type 2 diabetes mellitus, diabetic polyneuropathy; dry eye disease
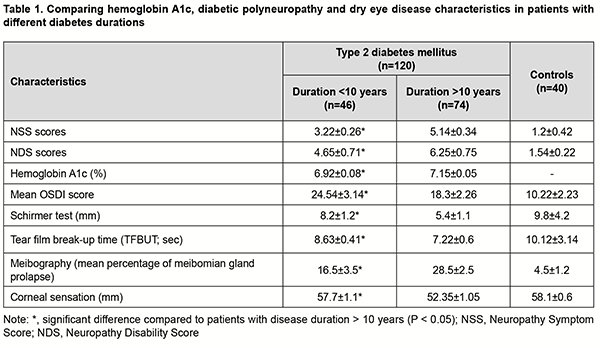
Introduction Almost 425 million people are affected by the global burden of diabetes mellitus, and this is predicted to increase to 629 million by 2045 [1]. Metabolic abnormalities in the body are the basis for the development of complications of DM, particularly such major ones as ocular complications, diabetic retinopathy and dry eye disease (DED) [2, 3]. When it comes to the ocular lesions in diabetes mellitus, the focus of attention is placed on diabetic retinopathy, whereas the manifestations of DED in diabetics are given little attention in the literature. However, DED is one of the most common chronic ocular surface diseases, and has been found in 35%-55% patients with DM [4, 5, 6]. DM is a systemic disease characterized by chronic hyperglycemia and impaired metabolism leading to lacrimal functional unit (LFU) dysfunctions varying in pathogenetic orientation. There have been reports that patients with DM exhibited corneal and conjunctival structural, metabolic and functional abnormalities, contributing to further progression of diabetic ocular surface complications [7, 8, 9]. It has been demonstrated that the risk of developing proliferative diabetic retinopathy and diabetic retinopathy increased with the duration of DM, and it is likely that the structural and functional parameters of the lacrimal functional unit in DED also undergo changes with progression of DM. In this connection, an objective quantitative study of the features of DED, with the identification of causative components in patients with progression of DM, was deemed especially relevant. The purpose of the study was to assess the state of the lacrimal functional unit in diabetic patients with dry eye and different durations of diabetes and different glycemic status. Material and Methods This study was conducted at the Department of Ophthalmology of the Republican Specialized Scientific and Practical Medical Center of Endocrinology. One hundred and twenty patients with both type 2 DM and DED were included in the study. There were 79 women and 41 men, mean patient age was 53.5 ± 9.6 years, and diabetes duration ranged from 3 years to 24 years. Patients with concomitant inflammatory and degenerative diseases of the ocular surface (conjunctivitis, keratitis, scleritis and pterygium) were excluded from the study. Patients were divided into two groups based on diabetes duration: Group 1 (DM duration less than 10 years; n=46) and Group 2 (DM duration more than 10 years; n=74). The control group included 40 healthy individuals. The groups were matched with regard to age, gender and compensation of DM. Hemoglobin A1c (glycated hemoglobin) levels and clinical manifestations of diabetic polyneuropathy (DPN) and DED were assessed. Hemoglobin A1c (glycated hemoglobin) levels were assessed using a routine methodology to determine the glycemic status. The Neuropathy Symptom Score (NSS) [7] was used for self-assessment of individual neuropathy sensory symptoms identified frequently by patients with DPN (prickling and/or tingling sensation; burning sensation; numbness; aching pain; convulsions; and allodynia). Sensation was scored as 0 = absent, 1 = sensation present; and 2 = sensation increases at night. The Neuropathy Disability Score (NDS) [8] was used for self-assessment of sensation severity; this score has been suggested for the comprehensive assessment of the peripheral nervous system. Because the NDS is cumbersome, we have used only the portion relevant to sensory loss assessment. Polyneuropathy was considered unlikely if the score for both legs was less than 2. A score of 3 to 5 corresponded to mild neuropathy; 6 to 8, to moderate neuropathy; and 9 to 10, to severe neuropathy (sensory deficiency). The Cochet–Bonnet esthesiometer was used to evaluate corneal sensation [7]. It consists of a fine nylon filament, the length of which can be adjusted from 5 mm to 60 mm to apply different intensities of stimuli, and the pressure exerted on the cornea increases with a decrease in the length. Dry eye severity was assessed using Ocular Surface Disease Index (OSDI) scores, Schirmer test (basal tear production), and Tear Film Blow Out time (TFBUT) and Meibo modes with Huvitz Auto Ref/Keratometer HRK-9000 (South Korea). Statistical analysis was conducted using Microsoft Office 2019 software. Spearman correlation was used to assess the association between variables. The level of significance p < 0.05 was assumed. Results Table 1 presents the results of comprehensive studies of A1c, DPN and DED characteristics. Our study of subjective DPN symptoms using the NSS demonstrated that mean NSS scores were significantly (p < 0.05) higher in patients with diabetes duration of > 10 years than in patients with diabetes duration of < 10 years. In addition, mean NDS scores were higher in patients with diabetes duration of > 10 years than in patients with diabetes duration of < 10 years, and the difference in NDS scores between the two groups was also statistically significant. A similar tendency was observed for glycated hemoglobin (HbA1c). In group 1, mean HbA1c levels were stable, within a normal range, and indicated the achievement of the target HbA1c, at which the development of diabetic complications was unlikely, as opposed to group 2.
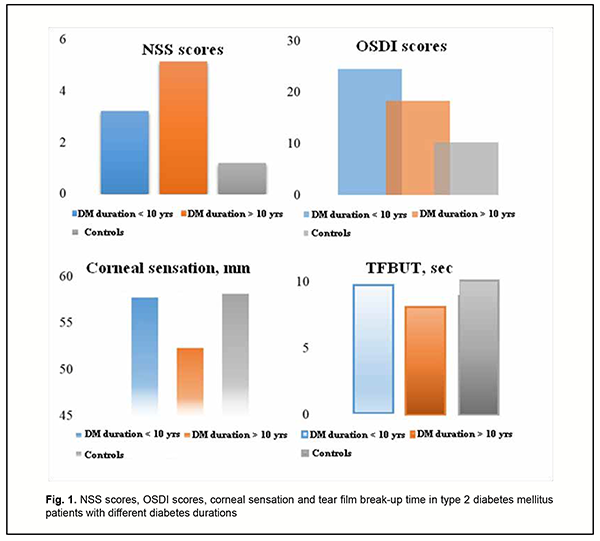
A negative correlation between diabetes duration and corneal sensation indicated that long-term hyperglycemia and metabolic syndrome in DM could decrease corneal sensitivity. OSDI scores were significantly higher in patients with diabetes duration of < 10 years than in patients with diabetes duration of > 10 years, and an amount of difference was usually of 5 to 6 points. Basal tear production as well as tear film break-up time (TFBUT) was significantly lower in patients with diabetes duration of > 10 years (Fig. 1).
Our meibography results demonstrated that there was an increase in the percentage of meibomian gland prolapse with an increase in diabetes duration. Thus, the percentage of those with meibomian gland dystrophy or meibomian gland prolapse was 1.5-times higher for group 1 than for group 2 (28.5 ± 2.5%; p < 0.05). Finally, corneal sensation as assessed by the esthesiometer was significantly (p < 0.05) lower in patients of group 1 than in those of group 2 or controls. Discussion There was a positive correlation of abnormalities in the structure and/or function of the lacrimal functional unit with glycated hemoglobin levels and DPN severity. Therefore, it can be argued that DED is a specific ocular complication of DM, because its development is pathogenetically associated with the structural changes in the ocular surface secondary to metabolic impairments [6, 11-13]. Although TFBUT and tear production were significantly lower in patients with diabetes duration of < 10 years than in patients with diabetes duration of > 10 years, subjective DED symptoms were more apparent in the former patients. This may be explained by a higher corneal sensation as assessed by the esthesiometer in patients with diabetes duration of < 10 years. Therefore, it was a decrease in corneal sensation in patients with diabetes duration of < 10 years that was a dominant factor for the course of DED in these patients. These findings confirm an important role of changes in the sensitive corneal nerve endings in the pathogenesis of DED in diabetics, which has been previously demonstrated in a study by Bikbov and Surkova [2] on confocal corneal microscopy in patients with diabetes. This is also in agreement with findings of Zeng and colleagues [8] who reported significant differences in TFBUT, tear production and subjective DED symptoms among patients with different durations of DM. Our findings suppose that there is an increase in neuropathy sensory symptoms identified by patients with an increase in DM duration. Although the lacrimal functional unit in general undergoes more apparent structural and functional changes (like meibomian gland prolapse and lesions in the auxiliary lacrimal glands), subjective DED symptoms become less apparent as the diabetic grows older. A decrease in corneal sensation in patients with diabetes results in a decrease in reflex tear secretion, whereas conjunctival neurodegeneration contributes to hypofunction of mucins (goblet cell-derived proteins), leading to abnormal tear-film quality and stability. Therefore, patients with long duration of DM have a latent course of DED and relatively severe structural abnormalities in the lacrimal functional unit. Subjective DED symptoms are biomarkers that diagnose this disease in early diabetes, whereas decreased dry eye symptoms in patients with long duration of DM indicates not only DNP progression, but also severe organic manifestations of corneal neuropathy. Therefore, our comprehensive studies of patients with diabetes mellitus found that diabetes duration plays a dominant role in the nature of the course of DED. The main features of DED manifestations in patients with DM are the predominance of the subjective symptoms in those with shorter diabetes duration and the absence of complaints of eye dryness as well as the presence of objective structural changes in the lacrimal functional unit in those with longer diabetes duration. Subsequently, ocular surface neuropathy contributes to metabolic abnormality progression in the cornea, and correspondingly causes degeneration of the corneal epithelium leading to corneal erosions. Therefore, this results in a vicious circle leading to an increase in LFU dysfunction and ocular surface damage in patients with long duration of DM. These clinical manifestations of DED are caused by lesions of the corneal nerve endings and reduced corneal sensation, and are ocular symptoms of DPN progression. A diabetic patient with neuropathy events requires a prophylactic examination by the ophthalmologist in order to reduce the risk of developing corneal degenerative lesions.
References 1.Azamatova GA, Aznabaev MT, Avkhadeeva SR. [Dry eye syndrome in patients with diabetes mellitus: prevalence, pathogenesis, clinical features. Meditsinskii vestnik Bashkortostana. 2018; 13(73):99-102. 2.Bikbov MM, Surkova VK. [Cornea and its changes in diabetes mellitus: the review]. Sakharnyi diabet. 2016;19(6):479-85. Russian. 3.Beckman KA. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea. 2014; 8(33):851–84. 4.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012 Jan;130(1):90-100. 5.Achtsidis V, Eleftheriadou I, Kozanidou E, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37(10):e210–e211. 6.Misra SL, Patel DV, McGhee CN, et al. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res. 2014;2014:848659. 7.Balashevich LI, Brzhevskii VV, Izmailov AS, et al. Ocular manifestations of diabetes. Moscow: Medicina; 2004. Russian. 8.Zeng X, Lv Y, Gu Z. The Effects of Diabetic Duration on Lacrimal Functional Unit in Patients with Type II Diabetes. J Ophthalmol. 2019 Jan 10;2019:8127515 9.Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004 Dec;18(2):168-74. 10.Neira-Zalentein W, Holopainen JM. Tervo TM, et al. Corneal sensitivity in diabetic patients subjected to retinal laser photocoagulation. Invest Ophthalmol Vis Sci. 2011 Jul 29;52(8):6043-9. 11.Pritchard N, Edwards K, Vagenas D, et al. Corneal sensitivity as an ophthalmic marker of diabetic neuropathy. Optom Vis Sci. 2010 Dec; 87(12):1003–8. 12.Eissa IM, Khalil NM, El-Gendy HA. A controlled study on the correlation between tear film volume and tear film stability in diabetic patients. J Ophthalmol. 2016;2016:5465272. Crossref PubMed 13.Najafi L, Malek M, Valojerdi AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Complications. Sep-Oct 2013;27(5):459-62. The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|