J.ophthalmol.(Ukraine).2021;3:28-33.
|
http://doi.org/10.31288/oftalmolzh202132833 Received: 25 January 2021; Published on-line: 29 June 2021 Longitudinal visual functional recovery in compressive optic neuropathy in patients with primary pituitary microadenoma after endoscopic transnasal surgery K. S. Iegorova 1, M. O. Guk 1, L.D. Pichkur 1, L.V. Zadoianyi 1, V.N. Konakh 2 1 SI «Romodanov Neurosurgery Institute of the National Academy of Medical Sciences of Ukraine»; Kyiv (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE:Iegorova KS, Guk MO, Pichkur LD, Zadoianyi LV, Konakh VN. Longitudinal visual functional recovery in compressive optic neuropathy in patients with primary pituitary microadenoma after endoscopic transnasal surgery. J.ophthalmol.(Ukraine).2021;3:28-33. http://doi.org/10.31288/oftalmolzh202132833 Background: Neoplasms of the chiasmal and optic nerve region can result in compressive optic neuropathy with reduced visual acuity (VA), visual field defects and primary descending optic atrophy. Anterior visual pathway compression by the neoplasm is an absolute indication for surgical intervention. Purpose: To review the phases of visual functional recovery in compressive optic neuropathy in patients with primary pituitary microadenoma after endoscopic transnasal surgery. Material and Methods: We retrospectively reviewed the records of 225 patients who were treated for pituitary adenoma at the Romodanov Neurosurgery Institute from 2017 through 2019. All patients (450 eyes) had optic nerve/chiasm complex (ONCC) compression, reduced VA and/or visual field defects preoperatively and underwent surgical decompression of the ONCC. The time points for eye examination were day 1 or 2 after hospitalization (time point 0), and day 5, 6 or 7 (early postoperative examination; time point 1), month 1 (time point 2), month 3 (time point 3), month 6 (time point 4), and month 12 (time point 5) after surgery. Results: In the majority of patients, decompression of the ONCC resulted in an improvement in or recovery of vision. There was a significant difference (p < 0.05) in VA and mean defect (MD) between time point 1 (VA: 0.74 ± 0.02; MD: 7.85 ± 0.29 dB) and time point 3 (VA: 0.8 ± 0.01; MD: 6.37 ± 0.28 dB). Moreover, there was a significant difference in MD between time point 1 (7.85 ± 0.29 dB) and time point 2 (6.91 ± 0.28 dB), and between time point 2 and time point 4 (6.08 ± 0.28 dB). Conclusion: After surgical decompression of the ONCC, we observed the two phases of visual functional recovery: fast (as long as several days) and delayed (until 6 months). There was practically no further improvement in visual functions after month 6. Keywords: skull-base tumors, pituitary asenoma, chiasmal syndrome, compressive optic atrophy, phases of visual functional recovery, endoscopic transnasal surgery Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
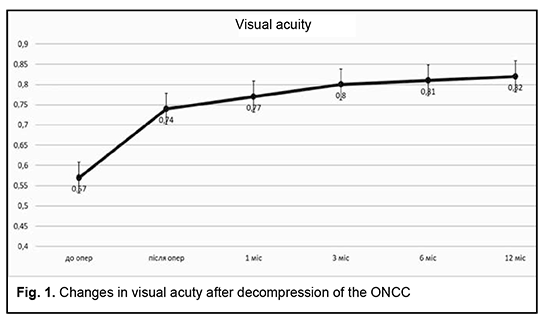
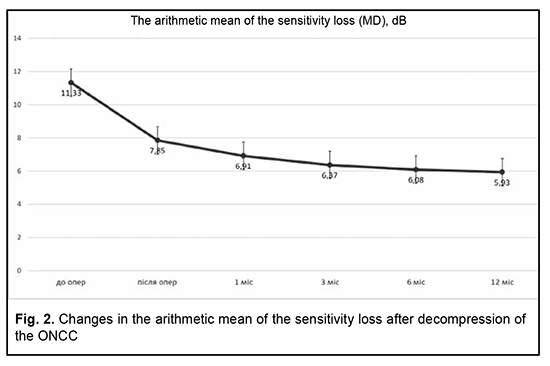
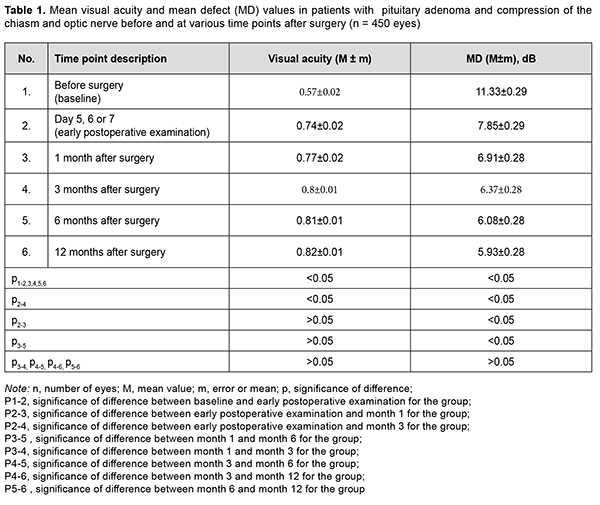
Introduction Compressive optic neuropathy (CON) in chiasmal tumors is accompanied by reduced visual acuity, bitemporal constriction of the visual fields, and development of descending compressive optic atrophy (OA). Impaired visual functions are observed in 67.8-83% of patients. Specifically, reduced visual acuity and visual field impairment have been found in 38-68.5% and 39.8-70%, respectively, of patients [1, 2, 3, 4]. 30-88.9% of cases manifest a loss of visual acuity or visual fields [1, 4, 5]. Prolonged chiasmal compression results in the development of OA in 26.7% to 72% of patients, leading to blindness in 3.5% to 25% of cases [5, 6, 7, 8]. Pituitary adenoma (PA), sella turcica meningioma and supradiaphragmatic craniopharyngioma are the most common neoplasms of the chiasmal and sellar region. Pituitary adenomas constitute 10% to 15% of all extracerebral intracranial tumors and are the most common benign primary intracranial tumors affecting the chiasm and leading to CON [5, 9, 10, 11]. Anterior visual pathway compression by the PA develops at the ophthalmological phase of the disease and is an absolute indication for surgical intervention. The gold standard of surgical treatment for PA is endoscopic transnasal excision of the lesion which aims to decompress the anterior visual pathway and improve or restore the visual function [11]. Studies of the last century indicated that the visual function may recover within a month, with a relatively high recovery rate immediately after decompression of the chiasm, and a lower recovery rate subsequently until month 1[12]. Recent studies, however, have demonstrated that the visual function may improve within up to three years of surgery, and this improvement can be divided into three phases: fast-recovery phase (several minutes to several days), delayed recovery phase (several weeks to several months), and late recovery phase (6 months to 3 years). Many researchers argue that the fast recovery phase occurs due to the recovery of the physiological conduction and improvement of axoplasmic flow in peripheral neuron fibers. The delayed and late recovery phases occur due to remyelination of nerve fibers [13, 14]. Longitudinal changes in visual function depend on patient’s age, baseline visual acuity and visual fields, duration of the compression of the visual pathway, presence of the optic atrophy, and size of the tumor [10, 15]. It is the endoscopic skull-base surgery that has been reported to be the best option for visual recovery [11]. However, standard ophthalmological follow-up time points after endoscopic skull-base surgery have not been specified [15, 16, 17]. Therefore, there is no clear idea of longitudinal changes in visual functions at the time points after surgical decompression of the optic nerve/chiasm complex (ONCC). The purpose of the study was to review the phases of postoperative visual function recovery in compressive optic neuropathy in patients with primary pituitary microadenoma after endoscopic transnasal surgery. Material and Methods We retrospectively reviewed the records of 225 patients (116 (51.6%) women and 109 (48.4%) men; aged 19 to 77 years; mean age, 51.3 ± 1.9 years) who underwent primary endoscopic transnasal surgery for histologically confirmed pituitary adenoma at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, from 2017 through 2019. The catamnestic period for these patients was at least 12 months. Exclusion criteria were continued tumor growth, signs of intracranial hypertension, ocular comorbidity, or drug (cabergoline)-induced decompression. Preoperatively, patients exhibited the compression of the ONCC and reduced visual acuity and/or visual field defects. They underwent clinical and neurological, eye, and otoneurological examination (a routine otoneurological examination with assessment of cranial nerve function) before and after treatment. The time points for examination were day 1 or 2 after hospitalization (time point 0), and day 5, 6 or 7 (early postoperative examination; time point 1), month 1 (time point 2), month 3 (time point 3), month 6 (time point 4), and month 12 (time point 5) after surgery. The monitoring at time points within a year was believed to be appropriate because there have been reports on delayed visual function recovery in compressive optic neuropathy in patients after endoscopic transnasal surgery for primary pituitary microadenoma. Instrumental and laboratory studies were conducted. Neuroimaging studies included computed tomography (CT) and contrast magnetic resonance imaging (MRI) of the brain. Neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, static automated and kinetic perimetry, and direct and indirect ophthalmoscopy. Static automated perimetry (SAP) was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) to localize visual field defects, with the arithmetic mean of the sensitivity loss, the mean defect (MD), being used to assess visual field loss severity. The visual field loss was classified as very severe if it was not possible to assess visual fields due to the extremely poor visual function. This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. Results are presented as the mean and standard deviation (M ± m). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ≤ 0.05 was assumed. Results Preoperatively, in each case, there was evidence of tumor growth towards the ONCC, which caused anterior visual pathway compression leading to visual abnormalities like loss of visual acuity and/or loss of visual fields, and these abnormalities were the major clinical findings in most patients. The duration of the visual abnormality ranged from one week to five years, with most patients exhibiting a gradual reduction in visual acuity and/or visual field. It is noteworthy that 23 patients (10.2%) had no visual complaints, and a loss of visual acuity and/or loss of visual fields in their eyes were detected preoperatively. Of the 225 adenomas, 190 (84.5%) were non-functioning, and 35 (15.5%) were functioning. In 31 patients (13.8%), both eyes had a visual acuity of 1.0, and, in 194 patients (86.2%), one or both eyes had reduced visual acuity. Best-corrected visual acuity was normal (1.0) in 123 (27.3%) eyes, mildly impaired (0.7-0.9) in 89 (19.8%) eyes, moderately impaired (0.4-0.6) in 86 (19.1%) eyes, severely impaired (0.1-0.3) in 94 (20.9%) eyes, and very severely impaired (< 0.1) in 40 (8.9%) eyes. In addition, 18 eyes (4%) were blind and 2 patients were bilaterally blind. Static perimetry found no changes in 16 (3.6%) eyes. Visual defects were observed in 434 eyes (96.4%). Temporal hemianopia (either complete or partial) only was the commonest field defect (210 eyes; 48.4%), followed by temporal hemianopia with central scotoma (128 eyes; 29.5%), central scotoma only (29 eyes; 6.7%), and residual visual field in the nasal inner quadrant, with a complete loss of central vision (34 eyes; 7.8%), and homonymous hemianopia (16 eyes; 3.7%). In addition, visual field was not measurable due to extremely low visual function in 17 (3.9%) eyes. The arithmetic mean of the sensitivity loss, the mean defect (MD), for the study patients was 11.33 ± 0.29 dB. Ophthalmoscopy found primary descending OA in 141 (62.7%) patients. Of these, 97 patients (194 eyes) exhibited bilateral OA, and 44 patients (44 eyes), unilateral OA. Symmetric, asymmetric and markedly asymmetric chiasmal syndrome was found in 100 (44.4%), 82 (36.4%), and 43 (19.1%) patients, respectively. That is, symmetric chiasmal syndrome was most common, indicating an injury to crossed optic nerve fibers, which is associated with the predominance of pituitary lesions with suprasellar symmetric extension towards the ONCC. Mean visual acuity and MD values for study patients at the time points are presented in Table 1 and Figures 1 and 2.
At the first postoperative time point (day 5, 6 or 7 after decompression of the ONCC), visual acuity was still 1.0 in 123 eyes (27.3%), and improved to 1.0 in 89 eyes (19.8%). In addition, the number of eyes with mildly impaired (0.7-0.9) visual acuity increased to 97 (21.6%), whereas the numbers of eyes with moderately impaired (0.4-0.6), severely impaired (0.1-0.3) and very severely impaired (< 0.1) visual acuity and amaurosis decreased to 62 (13.8%), 48 (10.7%) 21 (4.7%), and 10 (2.2%), respectively. There was a significant difference (p < 0.05) in visual acuity between pre-treatment (0.57 ± 0.02) and post-treatment (0.74 ± 0.02) values. Visual fields normalized in 84 eyes (18.7%), remained stable in 16 eyes (3.5%), and 350 eyes (77.8%) had visual field defects. Temporal hemianopia only was mostly either partial or relative, and was still the commonest field defect (211 eyes; 46.9%), followed by temporal hemianopia with central scotoma (67 eyes; 14.9%), central scotoma only (26 eyes; 5.8%), residual visual field in the nasal inner quadrant, with a complete loss of central vision (21 eyes; 4.7%), and homonymous hemianopia (14 eyes; 3.1%). In addition, visual field was not measurable due to extremely low visual function in 11 (2.4%) eyes. There was a significant difference (p < 0.05) in MD between time point 0 (before treatment; 11.33 ± 0.29 dB) and time point 1 (day 5, 6 or 7 after surgery; 7.85 ± 0.29). In addition, there was a significant difference (p < 0.05) in visual acuity (0.74 ± 0.02 and 0.8 ± 0.01, respectively) and MD (7.85 ± 0.29 dB and 6.37 ± 0.28 dB, respectively) between time point 1 (day 5, 6 or 7 after surgery) and time point 3 (month 3 after surgery). Although visual acuity further improved after month 3, the improvement was not statistically significant (p > 0.05). Moreover, there was a significant difference (p < 0.05) in MD between time point 1 (day 5, 6 or 7 after surgery; 7.85 ± 0.29 dB) and time point 2 (month 1 after surgery; 6.91 ± 0.28 dB), and between time point 2 time point 4 (month 6 after surgery; 6.08 ± 0.28 dB).
Discussion Pathogenetical mechanisms of CON are associated with direct compression of the ONCC and severely impaired conduction in neuron fibers, which causes optic nerve parabiosis. In the majority of patients, the improvement in or recovery of visual function occurs as a result of decompression, and depends on several factors like the chiasmal compression duration, type of disease course (stroke-like or tumor-like), tumor density, and presence of optic atrophy, which is in agreement with findings of others [10, 15]. Findings of studies by Findlay and colleagues (1983) [18] and Marcus and colleagues (1991) [19] as well as our findings suppose that fast visual function recovery in the early postoperative period occurs due to the removal of the restriction of the visual functions, improvement in axoplasmic flow and recovery of the physiological conduction [18, 19]. In the current study, at month 1 after decompression of the ONCC, the relative improvement in MD was not statistically significant. However, in a study by Kerrison and colleagues (2000) [20], the relative improvement in MD at visit 2 (1 month to 4 months) was statistically significant. In addition, they [20] concluded that a late phase (6 months to 3 years) of mild improvement may be marked in some individuals, which is likely to be a continuation of the fast recovery. Therefore, this is in agreement with our findings. A probable improvement in visual acuity and MD 3 months after decompression indicates the delayed slow recovery phase in the current study, which is not in agreement with findings from Trone (1955) and Serova (2011) [8, 11]. They used the method of kinetic perimetry, which is less sensitive than automatic static perimetry. An improvement does occur at 6 months to one year, but it is not statistically significant, which is comparable with the findings by Kerrison and colleagues (2000) [20], and is likely to be associated with improved patient concentration and trainability in the course of the study [20]. Therefore, in our opinion, the most profound improvement in visual functions after endoscopic transnasal surgery for chiasmal and optic nerve decompression in pituitary adenoma occurs during fast-recovery phase (several minutes to several days) and delayed recovery phase (several weeks to 6 months).
References 1.Foroozan R. Chiasmal syndromes. Curr Opin Ophthalmol. 2003; 14(6):325-1. 2.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 3.Wadud SA, Ahmed S, Choudhury N, Chowdhury D. Evaluation of ophthalmic manifestations in patients with intracranial tumours. Mymensingh Med J. 2014 Apr; 23(2):268-71. 4.Sefi-Yurdakul N. Visual findings as primary manifestations in patients with intracranial tumors. Int J Ophthalmol. 2015 Aug 18; 8(4):800-3. DOI: 10.3980/j.issn.2222-3959.2015.04.28. 5.Guk M.O. [Diagnosis and comprehensive treatment of hormonally-inactive pituitary adenomas]. Dissertation for Doctor of Medical Sciences. Kyiv; 2017. Ukrainian. 6.Masaya-anon P, Lorpattanakasem J. Intracranial tumors affecting visual system: 5-year review in Prasat Neurological Institute. J Med Assoc Thai. 2008; 91(4):515-9. 7.Tagoe NN, Essuman V A, Fordjuor G, Akpalu G, Bankah P, Ndanu T. Neuro-ophthalmic and clinical characteristics of brain tumours in a tertiary hospital in Ghana. Ghana Med J. 2015; 49(3):181-6. 8.Serova NK. [Clinical neuroophthalmology. Neurosurgery aspects]. Tver’: Triada; 2011. Russian. 9.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015; 76(3):210-9. 10.Lee DK, Sung MS, Park SW. Factors Influencing Visual Field Recovery after Transsphenoidal Resection of a Pituitary Adenoma. Korean J. Ophthalmol. 2018; 32(6): 488-96. 11.Wang EW, Zanation AM, Gardner PA, et al. ICAR: endoscopic skull-base surgery. Int. Forum Allergy Rhinol. 2019 Jul;9(S3): S145-S365. 12.Tron EZh. [Visual pathway disorders]. Moscow: Medgiz; 1955. Russian. 13.Kayan A, Earl CJ. Compressive lesions of the optic nerves and chiasm: pattern of recovery of vision following surgical treatment. Brain. 1975 Mar; 98 (1): 13-28. 14.McDonald WI. The symptomology of tumours of the anterior visual pathways. Can J Neurol Sci. 1982;9:381–90. 15.Peter M, De Tribolet N. Visual outcome after transsphenoidal surgery for pituitary adenomas. Br J Neurosurg. 1995;9:151–7. 16.Powell M. Recovery of vision following transsphenoidal surgery for pituitary adenomas. Br J Neurosurg. 1995;9:367–73. 17.Jakobsson K, Petruson B, Lindblom B. Dynamics of visual improvement following chiasmal decompression. Quantitative pre- and postoperative observations. Acta Ophthalmol Scand. 2002 Oct;80(5):512-6. 18.Findlay G, McFadzean RM, Teasdale G. Recovery of visionfollowing treatment of pituitary tumours: application of a new system of assessment to patients treated by transsphenoidal operation. Acta Neurochir. 1983;68:175–186. 19.Marcus M, Vitale S, Calvert PC, Miller NR. Visual parameters in patients with pituitary adenoma before and after transsphenoidal surgery. Aust NZ J Ophthalmol. 1991;19:111–8. 20.Kerrisson JB, Lynn MJ, Baer CA, Newman SA, Biousse V, Newman NJ. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol. 2000; 130(6): 813-20.
|