J.ophthalmol.(Ukraine).2021;6:3-7.
|
http://doi.org/10.31288/oftalmolzh2021637 Received: 28 June 2021; Published on-line: 21 December 2021 Exploring the features of keratometry readings in premature children I. A. Soboleva1, Y. Y. Borysenko2 1 Kharkiv Medical Academy of Postgraduate Education; Kharkiv (Ukraine) 2 Hirshman Municipal Clinical Hospital No.14, Kharkiv City Council; Kharkiv (Ukraine) E-mail: aps142d@gmail.com TO CITE THIS ARTICLE: Soboleva IA, Borysenko YY. Exploring the features of keratometry readings in premature children. J.ophthalmol.(Ukraine).2021;6:3-7. http://doi.org/10.31288/oftalmolzh2021637 Background: It is important for pediatric ophthalmologists to explore the keratometry parameters used in selection of contact lenses (and thus to improve visual function, quality of life, and treatment of complications of refractive errors) in premature children who received laser photocoagulation (LPC) to the avascular retina for threshold retinopathy of prematurity (ROP). Purpose: To assess the keratometry parameters in premature children who received LPC to the avascular retina for threshold ROP for the advanced improvement of visual functions and advanced treatment of complications of refractive errors in this category of patients. Material: We retrospectively examined the medical records (including automatic keratometry results) of 282 children (564 eyes). Results: There was a significant difference in corneal refractive power (CRP) in the steepest principal meridian and the flattest principal meridian between children of group 1 (i.e., premature children who received bilateral LPC) and group 2 (i.e., premature children who did not develop threshold ROP by the time retinal vascularization was complete) (р=0.00007 and р=0.0001, respectively) and between children of group 1 and group 3 (randomly selected full-term children) (р=0.000002 and р=0.000001, respectively), and mean CRP was higher in children who received LPC than in those who received no LPC. A significant difference in CRP between the steepest principal meridian and the flattest principal meridian (р=0.015) was found only for the comparison between the children who received LPC and controls. The value of this parameter was larger in children who received LPC than in full-term children. Conclusion: These findings may be helpful when selecting optical correction (e.g., orthokeratology correction) for refractive errors in the premature children who received LPC. Keywords: retinopathy of prematurity, kerastometry, contact lens correction
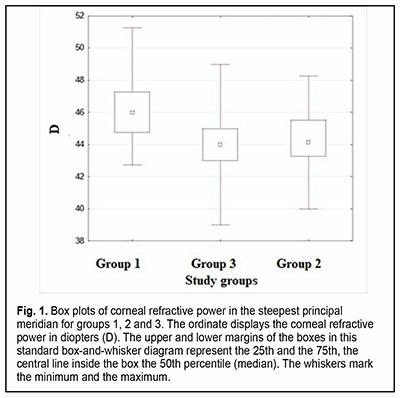
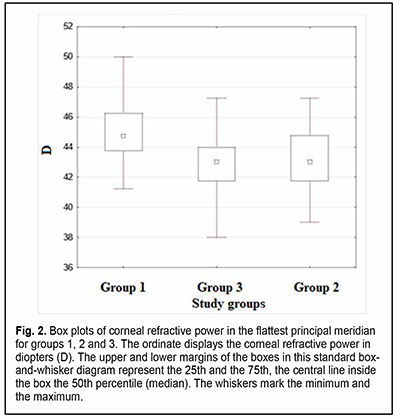
Introduction The optical system of the eye develops in a manner that facilitates obtaining the ratio of the axial length and corneal curvature which is favorable for achieving the best possible vision [1]. Ametropia is the refractive condition in which, with accommodation relaxed, parallel rays do not focus on the retina. In ametropia, a blurred image is created on the retina [2]. Astigmatism and other optical errors are conditions of refraction in which rays emanating from a single luminous point are not focused at a single point by an optical system. Total refractive astigmatism can be divided into corneal (or keratometric) astigmatism, lenticular astigmatism, and retinal astigmatism. Most astigmatism comes from the cornea [2]. The fact that refractive errors are common in premature children makes the use of means of optical correction for refractive errors a concern. Contact lenses are increasingly used in the pediatric ophthalmology practice, and, taking into account the above considerations, methods of contact lens correction for refractive errors deserve special attention. This type of optical correction is becoming increasingly popular not only because of the necessity of correction for refractive errors per se (which is more characteristic for adults than for children), but also to prevent really severe diseases like anisometropic amblyopia, refractive amblyopia and strabismic amblyopia. In addition, contact lens correction is sometimes an essential treatment for patients with diagnosed amblyopia, because correction with spectacles may be inadequate for correcting ametropia. The patient’s keratometry readings, horizontal and vertical visible iris diameters, basic corneal curvature and pupil size are usually important for contact lens selection [3, 4]. Optical correction for refractive errors with orthokeratology lenses has become increasingly popular recently. Orthokeratology (OK) is a clinical technique that uses specially designed rigid contact lenses to reshape the cornea to temporarily reduce or eliminate refractive error [5]. Given the fact that the above conditions may lead to a significant deterioration of quality of life and even to visual disability, studies of the eye parameters influencing the use of contact lens correction for refractive errors in premature children are important for current pediatric ophthalmology. Corneal optics and corneal morphology studies are essential for contact lens selection and may include corneal slit lamp techniques (biomicroscopy with or without staining), keratometry (ophthalmometry), keratotopography, pachimetry, optical coherence tomography of the anterior segment, etc. One of the most common of these techniques is keratometry (ophthalmometry). Keratometry assesses the mean curvature of the anterior corneal surface [5] and corneal asphericity. Given the above, one can make the conclusion with regard to the amount of corneal refractive power [7]. Keratometry works on the principle of recording the image size reflected from a known-sized object. The keratometer projects four points or approximately a dozen of Placido rings (illuminated concentric rings) onto the cornea. Given the object size and distance from image to object, the radius of curvature of the cornea can be calculated [8]. The calculation of corneal radius assumes the cornea to be a sphere whose radius of curvature is the same at all points. But this assumption is true only for the central 4 mm of the cornea (the optical corneal zone) measured by the keratometer [9]. Readings can be expressed in millimeters (mm) or diopters (D) [10]. The principal meridian at which the central anterior corneal surface has the steepest corneal curvature is called a steep meridian, whereas the meridian orthogonal to it is called a flat meridian. Keratometry measurements are made in newborns and later in life to detect astigmatism, keratoglobus and keratoconus. In addition, keratometry is included in the list of procedures to be performed prior to laser vision correction [11]. The results of anterior corneal surface analysis are widely used for selection of correction with soft contact lenses or rigid contact lenses. These are becoming increasingly popular for correction of various pediatric refractive errors that are corneal in origin or associated with abnormal anteroposterior length. Proper consideration should be given to adequate selection of contact lens size, centration, and movement in order to minimize the impact of contact lenses on normal function of the eye. Keratometry or corneal topography is essential in the fitting of contact lenses. Given the above considerations, it is important to explore the keratometry parameters used in the selection of contact lenses (and thus to improve visual function and quality of life) in premature children. The purpose of the study was to compare the premature children who received laser photocoagulation (LPC) to the avascular retina for threshold retinopathy of prematurity (ROP), the premature children who did not develop threshold ROP, and full-term children, with regard to keratometry parameters. Material and Methods We retrospectively examined the medical records (including automatic keratometry results such as refractive power and radius of corneal curvature for both principal meridians of both eyes) of 282 children (564 eyes) aged from 2.8 years to 11.5 years (mean age, 5.7 ± 2.58 years) during the study period. Inclusion criteria were (1) premature children that received bilateral LPC to the avascular retina for threshold ROP, or (2) premature children who did not develop threshold ROP by the time retinal vascularization was complete, with no significant difference in age during the study, time of gestation or birth weight with the premature children that received LPC, or (3) randomly selected full-term children whose age during the study was matched with the age of the premature children with no diagnosed chorioretinal pathology. The study children were divided into three groups. Group 1 included 23 premature children (46 eyes) that received bilateral LPC to the avascular retina for threshold ROP. Group 2 included 23 premature children (46 eyes) who did not develop threshold ROP by the time retinal vascularization was complete. Group 3 (the control group) was formed from 236 full-term children (472 eyes), with their number allowing for minimization of the effect of their individual features on the statistical results. Children with the detected diseases (like inflammatory and/or cicatricial changes, etc.) that may affect the optical characteristics of the cornea, soft contact lens wearers, orthokeratology wearers, and children with a history of ocular surgery were not included in the study. Libre Office Calc 7.0.4 spreadsheet (The Document Foundation), Statistica 10.0 (StatSoft, Tulsa, OK), and SPSS 17.0 (SPSS Inc, Chicago, IL) software were used for statistical analysis. Spearman, Pearson or Kendall correlation analysis was used when appropriate. Normality tests included the Lilliefors test, Kolmogorov-Smirnov test and Shapiro-Wilk test. The Kruskal-Wallis test, Mann-Whitney U test and Student’s t test were used to assess continuous data. Data are presented as mean (M) and standard error of mean (m). Results In children of group 1, steep keratometry readings ranged from 42.75 D to 51.25 D (mean value, 46.27 ± 0.36 D; median value, 46 D) and flat keratometry readings, from 41.25 D to 50.0 D (mean value, 45.07 ± 0.47 D; median value, 45.75 D). In addition, corneal curvature radius in the steeper principal meridian ranged from 6.59 mm to 7.9 mm (mean value, 7.31 ± 0.07 mm), corneal curvature radius in the flatter principal meridian, from 6.69 mm to 8.1 mm (mean value, 7.5 ± 0.08 mm), and the difference in refractive power between the two meridians, from 0.25 D to 3.25 D (mean value, 1.28 ± 0.11 D). For patients of Group 1 only, there was a modest correlation between the corneal refractive power and the amount of corneal astigmatism (the Spearman correlation coefficient was -0.04), and patient age during the study ranged from 2.8 years to 11.5 years (mean age, 5.7 ± 0.53 years). In children of group 2, steep keratometry readings ranged from 40.0 D to 48.25 D (mean value, 44.20 ± 0.28 D; median value, 44,25 D) and flat keratometry readings, from 39.0 D to 47.25 D (mean value, 43.08 ± 0.28 D; median value, 43 D). In addition, corneal curvature radius in the steeper principal meridian ranged from 6.99 mm to 8.44 mm (mean value, 7.64 ± 0.05 mm), corneal curvature radius in the flatter principal meridian, from 7.13 mm to 8.67 mm (mean value, 7.86 ± 0.05 mm), and the difference in refractive power between the two meridians, from 0.25 D to 2.75 D (mean value, 1.12 ± 0.11 D). For patients of Group 2 only, there was a modest correlation between the corneal refractive power and the amount of corneal astigmatism (the Spearman correlation coefficient was -0.07), and patient age during the study ranged from 2.0 years to 10.6 years (mean age, 5.43 ± 0.45 years). In children of group 3, steep keratometry readings ranged from 39.0 D to 49.0 D (mean value, 43.96 ± 0.08 D; median value, 44 D) and flat keratometry readings, from 38.0 D to 47.25 D (mean value, 42.96 ± 0.08 D; median value, 43 D). In addition, corneal curvature radius in the steeper principal meridian ranged from 6.9 mm to 8.05 mm (mean value, 7.59 ± 0.04 mm), corneal curvature radius in the flatter principal meridian, from 7.25 mm to 8.48 mm (mean value, 7.78 ± 0.04 mm), and the difference in refractive power between the two meridians, from 0 D to 4.75 D (mean value, 1.0 ± 0.03 D). For patients of Group 3 only, there was a modest correlation between the corneal refractive power and the amount of corneal astigmatism (the Spearman correlation coefficient was 0.03), and patient age during the study ranged from 2.9 years to 11.5 years (mean age, 5.48 ± 0.52 years). Figs 1 and 2 present box plots of corneal refractive power in the steepest principal meridian and the flattest principal meridian, respectively, for groups 1, 2 and 3.
There was a significant difference in corneal refractive power in the steepest principal meridian and the flattest principal meridian between children of group 1 (i.e., children who received bilateral LPC) and group 2 (р=0.00007 and р=0.0001, respectively) and between children of group 1 and group 3 (р=0. 000002 and р=0. 000001, respectively), and mean corneal refractive power was higher in children who received bilateral LPC than in those who received no LPC. There was no significant difference in corneal refractive power in the steepest principal meridian or the flattest principal meridian between children of group 2 and group 3 (р=0.39 and р=0.33, respectively). A significant difference in corneal refractive power between the steepest principal meridian and the flattest principal meridian (р=0.015) was found only for the comparison between children who received bilateral LPC and controls. We found that the amount of corneal astigmatism was higher in children who received bilateral LPC than in full-term children. No statistical difference in keratometry readings between the right eye and the left eye was observed. Discussion The significant difference in keratometry readings found between children who received bilateral LPC and both premature and full-term children who received no LPC indicated that the former children had higher corneal refractive power both in the steepest principal meridian and the flattest principal meridian, and a greater difference in corneal refractive power between the steepest principal meridian and the flattest principal meridian compared to full-term children. There have been reports on studies of the optical characteristics of the cornea in premature children. Yang and colleagues found that there was a significantly higher prevalence and greater magnitude of astigmatism in eyes with laser-treated threshold ROP compared with full-term controls. In addition, the steeper vertical corneal curvature component contributes to the increased astigmatism in eyes with laser-treated ROP [12]. A study by Repka [13] confirmed that the normal development of corneal curvature is affected by preterm delivery producing less hypermetropia in the setting of mild ROP. Snir and colleagues [14] found that babies with mild ROP at the corrected age of 40 weeks had higher and steeper keratometric values than term babies, but these authors reported neither on the results of corneal examination in children with laser-treated threshold ROP no on corneal astigmatism. Baker and Tasman [15] evaluated keratometry readings in myopic adults with ROP and myopic adults who were born full-term, but these authors did not report on the impact of laser treatment on keratometry readings. Connoly and colleagues [16] compared the refractive outcome of eyes treated with cryotherapy for threshold ROP with eyes treated with LPC. Ecsedy and colleagues [17] evaluated eyes of premature patients 7 to 14 years of age and found that premature eyes with or without mild ROP have more higher-order corneal aberrations compared to the eyes of term-born children. Our present findings complete those of others, demonstrating the impact of LPC on keratometry readings in eyes with ROP. These findings may be taken into account when selecting optical correction (e.g., orthokeratology correction) for refractive errors in premature children. Because of (1) the identified features of keratometry readings in pediatric eyes that received laser treatment for threshold ROP and (2) increased use of orthokeratology lenses in such children, this study suggests the need for further studies of the parameters (like corneal thickness, anterior chamber depth, lens thickness, and ocular axial length) obtained from corneal topography and other measurements in prematurity children, and the impact of these parameters on the longitudinal development of the internal optical system of the eye.
References 1.Koretz JF, Rogot A, Kaufman PL. Physiological strategies for emmetropia. Trans Am Ophthalmol Soc. 1995;93:105-18; discussion 118-22. 2.Fincham EF. The proportion of ciliary muscular force required for accommodation. J Physiol. 1955; 128:99-112. 3.Rykov SO, Shargorodska IV, Barinov IuV, Denisuk LI. [Refractive and accommodative errors. Locally adapted evidence based guidelines]. Decree No.827 of the Ministry of Health of Ukraine dated December 8, 2015]. Ukrainian. 4.Rykov SO, Iaremenko NM, Kharchenko LB. [Comprehensive examination of the eye and vision. Locally adapted evidence based guidelines]. Kyiv: Derzhavnyi ekspertnyi tsentr Ministerstva okhorony zdorov’ia Ukrainy; 2019. Ukrainian. 5.Snir M, Friling R, Weinberger D, et al. Refraction and keratometry in 40 week old premature (corrected age) and term infants. Br J Ophthalmol. 2004;88:900-4. 6.Polishchuk OS. [Diagnostic evaluation of the eye using a keratometer: A Collection of works]. Kyiv: FNMI NTTU KPI; 2016. Ukrainian. 7.Zavgorodnia NG, Sarzhevska LE, Ivakhnenko OM. [Ocular anatomy. Ophthalmological examination techniques]. Zaporizhzhia: Zaporizhzhia State Medical University; 2017. Ukrainian. 8.Polishchuk OS, Koziar VV. [Selecting the optimal illumination source for keratometer]. [Proceedings of the international conference]. Warsaw, 2018. Polish. 9.Sokurenko VM, Tymchyk GC, Chyzh IG. [Human eye and ophthalmic devices]. Kyiv: National Technical University of Ukraine ‘Igor Sikorsky Kyiv Polytechnic Institute’; 2009. Ukrainian. 10.Polishchuk OS, Koziar VV. [Selecting the optimal variant for diagnostic projections on the eye in keratometry]. Biomedychna inzheneriia I tekhnologiia. 2018;1(1):86-91. Ukrainian. 11.Polishchuk OS, Koziar VV. [Keratometry as the first stage of IOL implantation]. [Proceedings of the 3rd International Conference on International Systems and Technologies in Medicine]. Kharkiv: Zhukovskyi National Aerospace University ‘Kharkiv Aviation Institute’; 2020. p. 208-10. Ukrainian. 12.Yang CS, Wang AG, Shih YF, et al. Astigmatism and biometric optic components of diode laser-treated threshold retinopathy of prematurity at 9 years of age. Eye (Lond). 2013 Mar;27(3):374-81. 13.Repka MX. Refraction and keratometry in premature infants. Br J Ophthalmol. 2004 Jul;88(7):853-4. 14.Swarbrick HA. Orthokeratology review and update. Clin Exp Optom. 2006 May;89(3):124-43. 15.Baker PS, Tasman W. Myopia in Adults with Retinopathy of Prematurity. Am J Ophthalmol. 2008 Jun;145(6):1090-4. 16.Connolly BP, Ng EY, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: Part 2. Refractive Outcome. Ophthalmology. 2002 May;109(5):936-41. 17.Ecsedy M, Kovasc I. Scheimpflug imaging for long-term evaluation of optical components in Hungarian children with a history of preterm birth. J Pediatr Ophthalmol Strabismus. 2014 Jul 1;51(4):235-41.
Disclaimer: The authors state that the views expressed in this article are their personal and not the official positions of the institutions. Sources of Support: no. Conflict of Interest: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|