J.ophthalmol.(Ukraine).2021;6:73-76.
|
http://doi.org/10.31288/oftalmolzh202167376 Received: 20 June 2021; Published on-line: 21 December 2021 A case of ocular lesions in a patient with both tick borne borreliosis and toxoplasmosis V. M. Sakovych 1, S. B. Ustymenko 2, L. G. Berezniuk 2, N. A. Garkava 1 1 Dnipro State Medical University; Dnipro (Ukraine) 2 Dnipro Regional Eye Hospital; Dnipro (Ukraine) E-mail: garkava@ua.fm TO CITE THIS ARTICLE: Sakovych VM, Ustymenko SB, Berezniuk LG, Garkava NA. A case of ocular lesions in a patient with both tick borne borreliosis and toxoplasmosis. J.ophthalmol.(Ukraine). 2021;6:73-76. http://doi.org/10.31288/oftalmolzh202167376
Background: The prevalence of tick borne borreliosis has increased significantly in recent years. There have been reports on conjunctivitis, anterior uveitis, optic neuritis and other inflammatory eye diseases caused by Borrelia species. Case: This case of successful treatment of focal chorioretinitis caused by Borrelia burgdorferi/Toxoplasma gondii co-infection indicates that elucidating etiology and administering etiological therapy are essential for successful treatment outcomes. Discussion: Since there are few reports in the literature describing the ocular manifestations of Lyme disease, borreliosis is infrequently suspected by ophthalmologists as an etiologic factor in the development of inflammatory eye disease. Consequently, an enzyme immunoassay for Borrelia burgdorferi is not used in a routine diagnostic evaluation of patients with inflammatory eye disease. Conclusion: Specific Borrelia burgdorferi antibody serology is required to elucidate the etiology of ocular inflammation if a patient has a history of tick bites even in the absence of symptoms of Lyme disease. In addition, one should take in account that ocular infections are frequently mixed in nature. Keywords: tick borne borreliosis, Lyme disease, chorioretinitis
Introduction Until recently, tick borne borreliosis (also known as Lyme disease and Lyme borreliosis) was believed to be a rather rare disease, but its prevalence has increased significantly in recent years due to an increase in Ixodes tick population [1]. Lyme borreliosis is a major zoonotic disease caused by the Ixodes tick-transmitted Borrelia species (a genus of bacteria of the spirochete phylum), primarily Borrelia burgdorferi, with animals serving as reservoirs of the disease [2, 3]. In Ukraine, natural foci of the disease are spread not only over the forest areas, but over practically the whole country [4, 5]. Lyme disease is most common in spring and autumn, which may be associated with a seasonal increase in the rate of visits to natural leisure areas as well as an increase in the seasonal activity of ticks in May to June and September to October [2, 4, 6]. The disease has a phasic course and is characterized by severe lesions involving various organs and systems. Lesions are most common in the skin, nervous and cardiovascular systems and joints [6-8]. Ocular lesions in tick borne borreliosis are rather uncommon. The most commonly reported ocular finding in Lyme disease is transient conjunctivitis, which occurs in as much as 11% of patients with stage I disease [3, 9]; however, this condition is frequently not diagnosed. In patients with chronic Lyme disease, conjunctivitis may have a chronic and recurrent course, and may be an important symptom of this infection [10]. Other early manifestations of ocular borreliosis may include neuro-ophthalmological disorders such as oculomotor nerve palsy and/or optic neuritis, which is associated with the penetration of the blood ocular barrier by spirochetes as early as seven days after primary infection [3, 11]. Keratitis, anterior uveitis, and, less frequently, choroiditis and panueveitis may develop in late Lyme disease. The pathogenesis of these disorders is believed to be associated with the immunopathogenic effect of the agent on the ocular tissues [6-8]. Case report A 38-year-old male patient presented with complaints of a several-week history of gradually decreasing visual acuity OS and a “spot” in front of his left eye. The patient did not associate his disease with any specific cause, had no previous history of eye disease and denied any chronic somatic disease, allergy or history of surgery. Further history revealed that he was a hunter, had a history of tick bites, but did not pay attention to them. On examination, the left eye had a visual acuity (VA) of 0.8 and did not improve with correction, whereas the right eye had a VA of 1.0. Peripheral visual fields were normal for both eyes, but there were negative relative peripheral scotomata OD and positive relative central and paracentral scotomata OS. Color perception was normal. Intraocular pressure (IOP) as assessed by Maklakoff tonometry was 18 mmHg OU. The anterior segment, optic media and optic disc were normal for both eyes. Fundus examination demonstrated areas of retinal edema near the optic disc and along the course of the lower vascular plexus in the right eye, and areas of retinal edema in the macula and paracentrally, as well as foci of retinal degeneration in the left eye. Optical coherence tomography (OCT) findings included elevated retinal pigment epithelium (RPE) in the temporal quadrant of the right eye and elevated RPE with a subretinal slit-like space centrally and in the temporal quadrant of the left eye (Fig. 1).
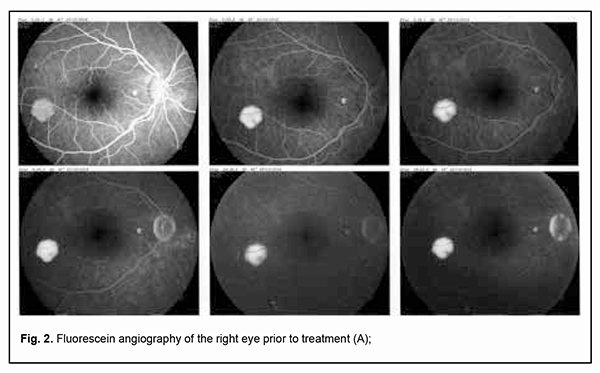
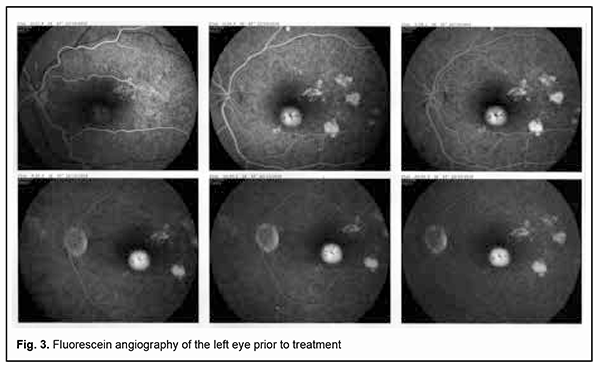
Fluorescein angiography (FA) revealed multiple leakage areas at the optic disc and along the course of the lower vascular plexus in the right eye (Fig. 2) and in the macula and paracentrally in the left eye (Fig. 3).
The patient was diagnosed with bilateral focal chorioretinitis. He underwent additional routine evaluation procedures to elucidate the etiology of the disease. His complete blood cell count, C-reactive protein, and rheumatoid factor were normal, and results of Mantoux test and Wassermann test negative. Evaluation by otorhinolaryngologist and dentist found no pathology. No DNA of Toxoplasma gondii, herpes simplex virus (HSV)-1 or HSV-2, or cytomegalovirus was found in the tear fluid. Tests for anti-Chlamydia trachomatis, anti-HSV-1, anti-HSV-2, anti-cytomegalovirus and anti-human immunodeficiency virus (HIV) by enzyme immunoassays were negative. Toxoplasma gondii IgG antibodies and Borrelia burgdorferi IgG antibodies were detected. The former antibodies were of low avidity. The patient was diagnosed with toxoplasmosis and borreliosis and prescribed a particular therapy by an infection specialist. He was treated on an inpatient basis at the eye hospital; particularly, he had retinal laser photocoagulation and a course of anti-inflammatory, anti-edematous, retinal protective and immunomodulating drug therapy. No positive changes were observed until the patient received his particular etiologic therapy. After the etiologic therapy for toxoplasmosis (oral trimethoprim-sulfamethoxazole, pyrimethamine and folic acid) and borreliosis (intramuscular ceftriaxone) was administered, complaints disappeared and patient’s visual functions recovered. Particularly, visual acuity improved to 1.0 OU, and the patient exhibited normal visual fields as assessed by perimetry. In addition, the retinal edema disappeared, and OCT as well as fluorescein angiography was normal for both eyes. Moreover, no worsening was observed throughout two years of follow-up. Discussion Since there are few reports in the literature describing the ocular manifestations of Lyme disease, borreliosis is infrequently suspected by ophthalmologists as an etiologic factor in the development of inflammatory eye disease. In addition, an enzyme immunoassay for Borrelia burgdorferi is not used in a routine diagnostic evaluation of patients with inflammatory eye disease. It has been reported that ocular manifestations develop in the presence of common symptoms of Lyme disease such as fever, joint pain and skin manifestations [3, 10]. It is of note that there were no extraocular manifestations of the disease in our patient. This case indicates that elucidating etiology and administering etiological therapy are essential for successful treatment outcomes. Conclusion Therefore, specific Borrelia burgdorferi antibody serology is required to elucidate the etiology of ocular inflammation if a patient has a history of tick bites even in the absence of symptoms of Lyme disease. One should take in account that ocular infections are frequently mixed in nature. Determining etiology and administering particular etiological therapy are essential for successful outcomes of treatment for infectious disease.
References 1.Yankovskaya YD, Chernobrovkina TY, Koshkin MI. [Status update on the problem of ixodic Lyme disease]. Archives of Internal Medicine. 2015; 6 (26): 21-27. Russian. 2.Bondarenko AV, Mogylenets OI, Katsapov DV. [Laboratory diagnosis of Ixodes tick-borne borreliosis]. In: [Proceedings of National Ukrainian Conference on the Current Aspects of Infectious Diseases in the Practice of an Internist]. Sumy, Ukraine, 15-16 June, 2016. p. 23-25. Ukrainian. 3.Loskutov IV. [Ocular manifestations and treatment of Lyme disease]. Rossiiskii meditsinskii zhurnal. 1997;5:8. Russian. 4.Chemych MD, Lutai IV. [Lyme disease: current state of the problem]. Skhidnoukrainskyi medychyi zhurnal. 2020;8(2):230-41. Ukrainian. 5.Shkilna MI, Andreichyn ML, Korda MM, Klishch IM, Zaporozhan SJ, Guk MT. [Lyme disease and other transmissible infections: diagnosis, treatment and prevention]. Aktualna Infektologiia. 2018; 5: 309-10. Ukrainian. 6.Sarkisan DS. [Ixodes tick-borne borreliosis: current state of the problem]. Infektsionnyie bolezni. 2015; 13(2):61-7. Russian. 7.Sarksian DS, Maleev VV, Platonov EV. [Differential diagnosis of Ixodes tick-borne borreliosis caused by Borrelia miyamotoi]. Infektsionnyie bolezni. 2012; 10(4): 41-44. Russian. 8.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lyme borreliosis. Lancet. 2012 Feb 4;379(9814):461-73. 9.Brouqui P. Guidelines for the diagnosis of tick borne bacterial diseases in Europe. Clin Microbiol Infect. 2004 Dec;10(12):1108-32. 10.Krupinina VS, Utenkova E.O. [Ocular manifestations of tick-borne borreliosis]. Tochka zreniia. Vostok-Zapad. 2014; 2:60. Russian. 11.Shapiro ED. Lyme Disease. N Engl J Med. 2014;1:21-6. Conflict of Interest: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|