J.ophthalmol.(Ukraine).2021;6:31-40.
|
http://doi.org/10.31288/oftalmolzh202163140 Received: 10 September 2021; Published on-line: 21 December 2021
Longitudinal changes in neuroophthalmological abnormalities in patients who underwent surgery for skull-base epidermoid cysts V. O. Fedirko, K. S. Iegorova, O. M. Lisianyi, A. G. Naboichenko, P. M. Onishchenko, D. M. Tsiurupa, M. V. Iegorov, V. V. Shust, A. O. Lisianyi The State Institution "Romodanov Neurosurgery Institute, National Academy of Medical Sciences of Ukraine"; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE: Fedirko VO, Iegorova KS, Lisianyi OM, Naboichenko AG, Onishchenko PM, Tsiurupa DM, Iegorov MV, Shust VV, Lisianyi AO. Longitudinal changes in neuroophthalmological abnormalities in patients who underwent surgery for skull-base epidermoid cysts. J.ophthalmol.(Ukraine). 2021;6:31-40. http://doi.org/10.31288/oftalmolzh202163140 Background: Even if current technologies are used in removal of a skull-base epidermoid cyst, the maintenance of function of the cranial nerves is still an important issue. Purpose: To assess changes in ophthalmological abnormalities after surgery for skull base epidermoid cysts. Material and Methods: We retrospectively reviewed the medical records of 21 patients who underwent surgery for epidermoid skull base cysts and had either ophthalmological abnormalities (abnormal visual, ocular motor functions and/or abnormal blood supply to the eye) or an involvement of the cranial nerves (CN; i.e., the ophthalmic, ocular motor, trigeminal, abducens and/or facial nerves). The surgical strategy was to aim for total tumor removal in order to prevent the development of postoperative meningitis and reduce the risk of tumor recurrence. Cranial nerve manipulations were performed under the highest available microscope magnification and with the use of endoscope imaging. In addition, intraoperative monitoring of the relevant cranial nerves was performed. All surgical procedures were video recorded. Long-term outcomes of surgery were determined either by outpatient examination or via a phone call using a standard checklist. Results: Total removal was achieved in 7 patients (33.3%), near total removal in 2 patients (9.5%), and subtotal removal in 12 patients (57.2%). Ocular motor functions were normal after surgery in 11 patients (52.4%). In addition, abnormalities in the early postoperative period were observed in 10 patients (47.6%), but the functions subsequently normalized in 3 patients and improved but not normalized in 7 patients. All the three patients with trigeminal neuralgia showed regression of pain syndrome. Conclusion: We determined the ophthalmological abnormalities that had been present before and after surgery, and the time required for function recovery. We found that endoscopic-assisted radical removal of the epidermoid cyst under the highest available microscope magnification with intraoperative cranial nerve monitoring was safe and effective for preserving cranial nerve functions in the late time points after surgery. Keywords: skull-base epidermoid cysts, ophthalmological abnormalities
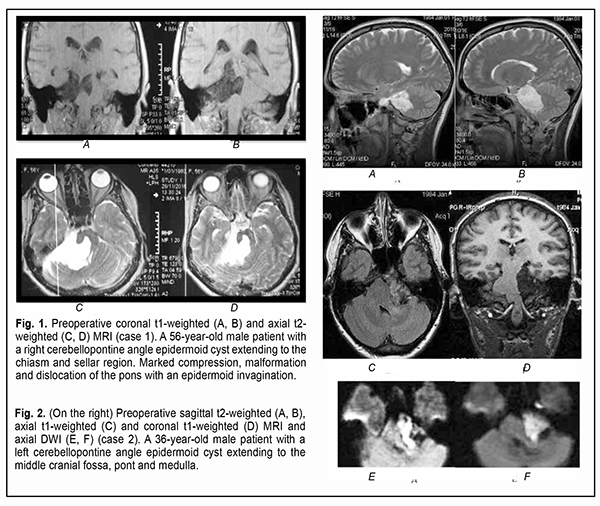
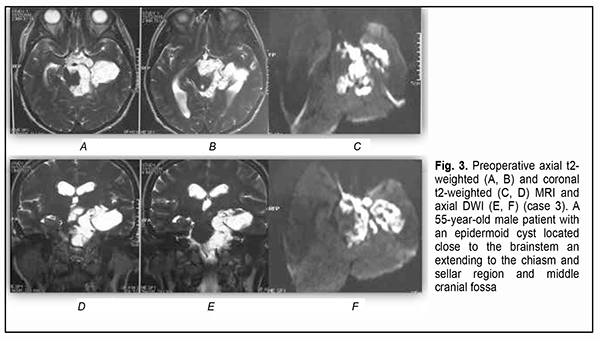
Introduction Epidermoid cysts manifest themselves clinically predominantly between the ages of 20 and 50 years, most commonly occur in the skull base, and usually arise in the subarachnoid cisterns, cerebellopontine angle and parasellar region. They grow slowly and can attain a large size before the onset of neurological symptoms. As the neoplasm grows, it gradually presses on, displaces and envelops the cranial nerves (CN) and adjacent vessels, and frequently invaginates the brainstem, causing ophthalmological abnormalities and lesions of relevant CN (Fig.1, 2, 3) [1, 2, 3].
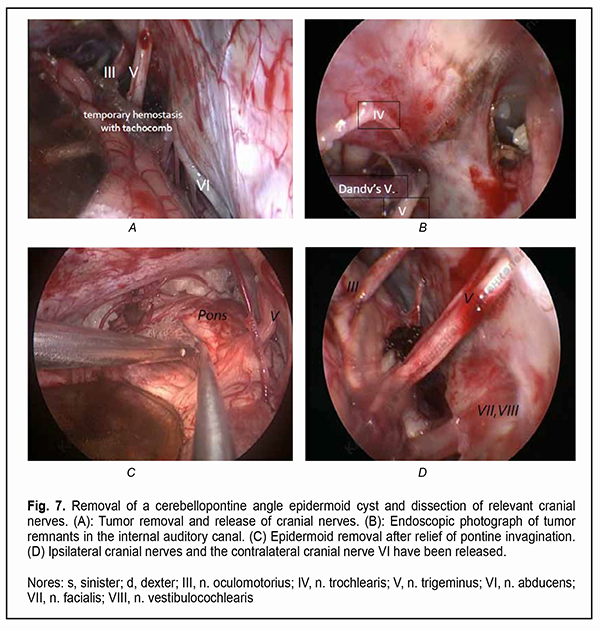
It is a common opinion that a total resection of the epidermoid cyst facilitates a prolonged period without relapse [4-10]. Incomplete cyst resection is associated with a significantly higher rate of epidermoid tumor recurrence compared to total resection [11, 12].. Since epidermoid cysts most commonly present at the fourth decade of life and recurrent symptoms can develop as late as 30-40 years after surgery, a total resection of the epidermoid cyst is not rational, especially in senile patients [10]. Others [13, 14, 15] reported a substantially shorter time to epidermoid recurrence, which is in agreement with our experience. On the one hand, because portions of the epidermoid capsule commonly become densely adherent to the surrounding neurovascular structures, making complete removal (and, consequently, achieving the maintenance of function of the cranial nerves, especially the ocular motor and ophthalmic nerves) challenging, a number of authors [11, 12, 16] advocate avoiding a total removal of the epidermoid cyst. Surgical intervention is usually aimed at making tumor excision as safe as possible, but dissecting the capsule away from the above nerves is challenging. Given the nature of tumor growth, the use of a neurosurgical microscope and an endoscopic approach and intraoperative monitoring of cranial nerves are necessary for the excision of skull base epidermoid cysts [10, 11, 17]. However, even a subtotal resection of the epidermoidal cyst may fail preventing aseptic meningitis with the cerebrospinal fluid characterized by negative bacterial cultures [18, 19]. Therefore, based on the literature, there is no unanimous opinion on whether an epidermoidal cyst should be totally removed or the adherent capsule should be left on the cranial nerves [5]. To the best of our knowledge, only a few cases of ophthalmological dysfunction in epidermoid cysts [20-26] and no data on the length of time required for recovery of visual and ocular motor functions after epidermoid removal have been reported. The purpose of the study was to assess longitudinal changes in neuro-ophthalmological abnormalities after surgery for skull base epidermoid cysts. Material and Methods We retrospectively reviewed the medical records of 21 patients (21 to 50 years; mean age, 35.2 ± 8.9 years) with skull base epidermoid cysts (predominantly, in the cerebellopontine angle or the parasellar region) who had either visual and ocular motor abnormalities or an involvement of the cranial nerves relevant to ophthalmological abnormalities, and had their cysts removed during the period from 2010 through 2020. Most patients (17/21) were of the young age (18-44 years), and the rest were of the middle age (45-59 years). The gender distribution was relatively equal (males constituted 42.85%). The House-Brackmann (HB) Grading System was used to assess the function of the facial nerve (CN VII), whereas the Barrow Neurological Institute Pain Scale (BNI-PS) and Barrow Neurological Institute Numbness Scale (BNI-NS), the function of the trigeminal nerve (CN V). Cyst location was determined based on preoperative T1w, T2w magnetic resonance imaging (MRI) and diffusion-weighted images (DWI) data. The location of the cranial nerves and their involvement in the neoplasm were verified using constructive interference in steady state 3 dimensional (CISS3D) on a 1.5-T Siemens MRI system or 3Ddrive on a 3.0-T Philips Achieva MRI system (Best, The Netherlands). Location of the epidermoid, location/involvement of the cranial nerves, presence and severity of adhesions of the cyst capsule to cranial nerves and/or the brainstem were assessed intra-operatively, which was video recorded by Sony HDR-PJ10 microscope camera and Karl Storz endoscope camera and documented in the protocol. A midline suboccipital and subtentorial approach was used for the epidermoid cysts of the fourth ventricle. Variations of the retrosigmoid approach used for the epidermoid cysts confined to the cerebellopontine angle depended on the extension of the epidermoid in the posterior fossa. Combined approaches were used if the lesion extended outside the posterior fossa into the middle fossa. The period from the onset of ophthalmological or neurological symptoms to surgery varied from one to 180 months. A neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, static automated and kinetic perimetry, and direct and indirect ophthalmoscopy. The amount of eye movement was determined for each of the four principal directions of gaze (as per Golovin) to determine the objective criteria for assessing the severity of the third, fourth and/or sixth cranial nerve lesions and changes in the recovery of oculomotor functions. The amount of eye movement was defined as normal (37° upward, 53° downward, 43° outward, or 46° inward), restricted (6° to 36° upward, 6° to 52° downward, 6° to 42° outward, or 6° to 45° inward), or no or almost no (0° to 5°) eye movement. The result of the study was presented as unimpaired ocular motility, restricted ocular motility or no ocular motility. Direct and consensual pupil response to light, and the size and symmetry of the pupils were assessed. Visual acuity was assessed with a diaphragm if there was mydriasis caused by CN III palsy. A Karl Zeiss OPMI MD stereo microscope equipped with Superlux 300 Fiber Optic Illumination System (Karl Zeiss Microscopy Deutschland GmbH, Oberkochen, Germany); a 4-mm/2.7-mm, 30-degree/45-degree KARL STORZ Hopkins II endoscope (Karl Storz GmbH & Co., Tuttlingen, Germany) and nerve integrity monitor (NIM-Response 3.0 System, Medtronic Xomed, Jacksonville, Florida) were used during epidermoid removal according to the routine methodology for the oculomotor nerves. Electrodes were placed on the CN III, IV, V, VI and VII, and switched over to the relevant nerve during a surgical procedure. The highest available microscope magnification (24х) was used while guiding the capsule away from the vessels, brainstem and nerves. In addition, an endoscope with a 30-degree or 45-degree viewing angle was used for examining the areas that would otherwise be difficult to view (Fig. 7).
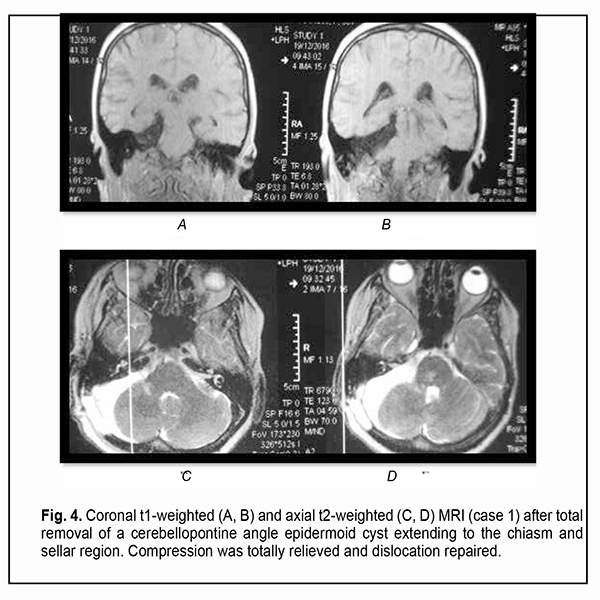
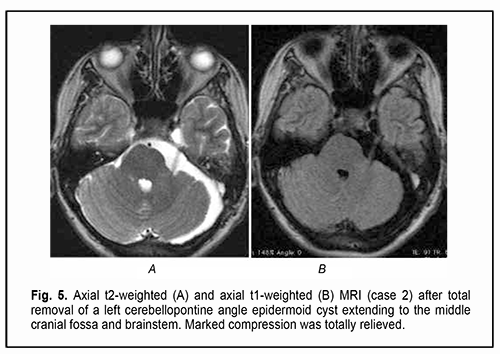
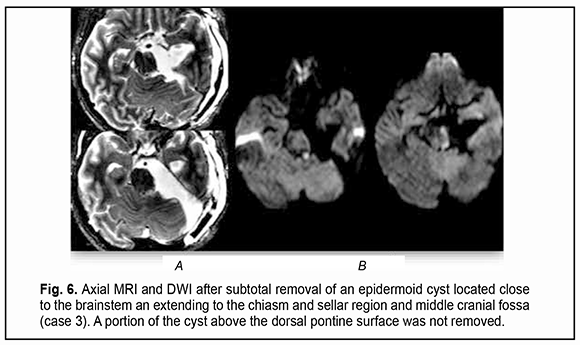
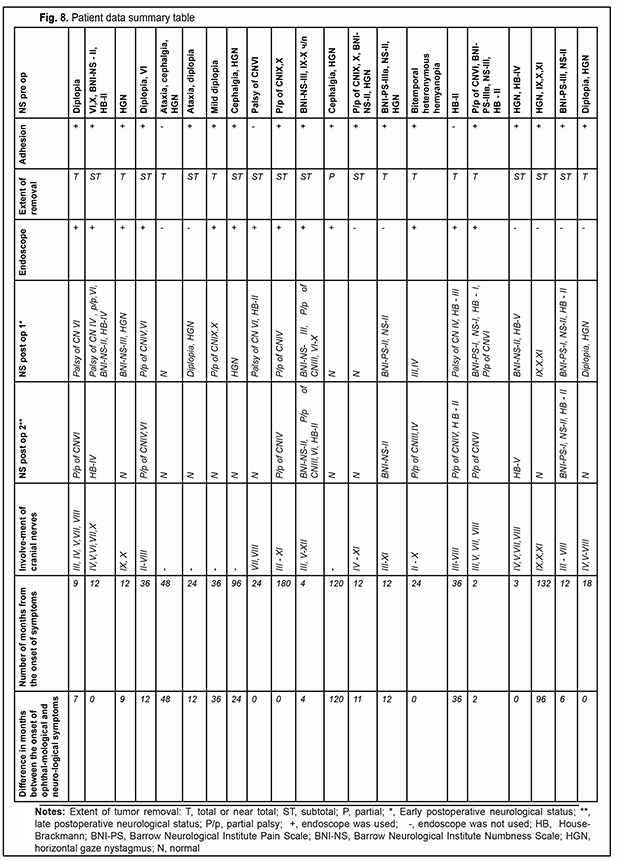
Of the 21 patients, 7 (33.33%) had a gross total removal and 2 (9.53%) had a near total removal of the cyst, with residual capsule fragments not exceeding 2-3 mm (Figs. 4, 5, 6, and 7). In addition, 12 (57.14%) had a subtotal removal of the cyst, with either intraoperative evidence of residual capsule fragments measuring from 2 mm to 10 mm, or postoperative MRI evidence of residual capsule fragments not exceeding 11 mm in the largest dimension. The capsule adhesion to surrounding neurocascular structure at the location where the dissection was difficult was seen in 18 patients (85.7%). In some cases, this can lead to the impossibility of safe total capsule dissection from the brainstem and cranial nerves. Postoperative follow-up ranged from 1 to 12 years, with mean and median values of 38.5 ± 4.8 months and 24 months, respectively (Fig. 8).
This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. Results are presented as the mean and standard deviation (M ± SD) and the percentage of the incidence of symptoms. Statistical analyses were conducted using Statistica 6.0 (StatSoft, Tulsa, OK, USA) software.
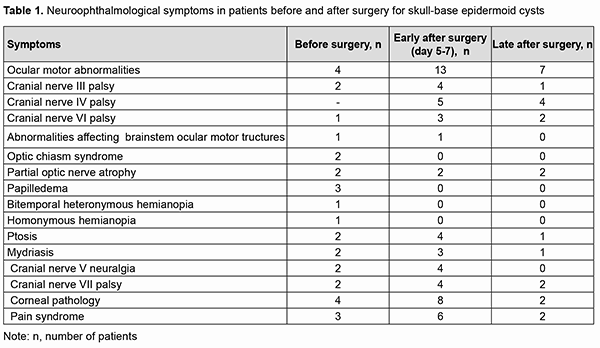
Results Of the 21 neoplasms, 7 (33.33%) were seen just to the right of the brainstem, including 2 (9.52%) that had a supratentorial extension; 9 (42.87%) were seen just to the left of the brainstem, including 3 (14.28%) that had a supratentorial extension; and 5 (23.8%) were located along the midline (cysts of the fourth ventricle). Of the 5 cysts (23.8%) that had a supratentorial extension, 2 (10%) extended to the middle and posterior fossa, with involvement of the chiasm and optic nerve. MRI-based neoplasm dimensions and volume varied from 24х22х18 mm до 54х57х44 mm and from 4.75 cm3 to 67 cm3, respectively, although volume estimation could not be accurate due to a complex shape and extension of epidermoids. Table 1 shows longitudinal changes in neuroophthalmological symptoms in patients who underwent surgical removal of a skull-base epidermoid cyst.
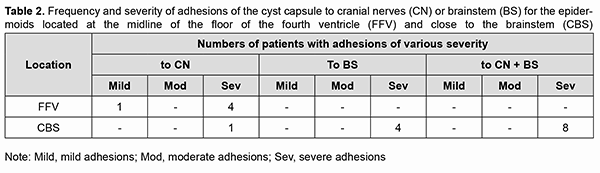
At presentation, ocular motor (OMA) were found in 4 patients (19.05%). Of these patients, 2 (9.52%) had isolated partial CN III (oculomotor nerve) palsy; one (4.76%), partial CN VI (abducens nerve) palsy; and one (4.76%), abnormalities affecting brain stem ocular motor structures (right and left gaze paresis). Patients with paralytic strabismus suffered from diplopia, dizziness, headache, nausea and instability of gait. In the two patients (9.52%) with isolated partial CN III palsy, there was compression of the optic nerve/chiasm complex, which was manifested by reduced visual acuity, descending optic atrophy and visual field defects (bitemporal heteronymous hemianopia with bitemporal paracentral scotoma in one patient and homonymous hemianopia with predominantly affected posterior chiasm in another patient). The isolated CN III lesion was manifested by the relevant ptosis, mydriasis, and restricted upward, downward and inward movements of the eye. The CN VI lesion was manifested by esotropia and a restricted outward movement of the eye. The isolated CN V (trigeminal nerve) lesion was found in 2 patients (9.52%) and accompanied by reduced corneal sensitivity and the development of neurotrophic keratitis. The CN VII (facial nerve) lesion was found in 2 patients (9.52%) and accompanied by lagophthalmos and the development of keratitis. It was dominating pain syndrome and trigeminal neuralgia, but not the initial presence of minor ophthalmological abnormalities that made 3 patients (14.28%) with involvement of several cranial nerves seeking medical care. Fundus examination revealed mild or moderate papilledema in three patients (14.28%). Of these patients, one had partial CN VI palsy and another had a brain stem associated ocular motor abnormality (OMA). Thirteen patients (61.9%; including the four who had had an OMA before surgery) had an OMA in the early postoperative period (days 5 to 7 after surgery): four had a CN III lesion (including the two in whom a CN III palsy appeared after surgery), five had a CN IV lesion, three had a CN VI lesion (including the two in whom a CN VI palsy appeared after surgery), and one had a brain stem nucleus abnormality. An OMA was present along with a CN V lesion in four patients (a lesion deteriorated to that of BNI-NS III in three of these four patients); along with a CN VI lesion in four patients (a CN VI palsy appeared in two of these four patients); and along with a lesion of CN IX and CN X in one patient. Both patients with preoperative compression of the optic nerve/chiasm complex showed recovery of visual acuity and visual fields due to decompression of the complex after surgery. Postoperatively, the papilledema regressed completely within a month in all the three patients with preoperative papilledema. In the late period (1-3 months) after surgery, CN III palsy was still present in 1 patient; CN IV palsy, in 4 patients; CN VI palsy, in 2 patients; and CN VII palsy, in 2 patients. Partial recovery of CN V function (with the development of keratitis and reduced corneal sensitivity) was observed in 4 patients, of whom 2 had had a preoperative CN V lesion, and the rest had postoperative dysfunction of CN V. The development of lagophthalmos and keratitis was assessed in the 4 patients with a CN VII lesion. Of these, the first patient was graded as HB II preoperatively and as HB IV postoperatively; the second, as HB I preoperatively and as HB II postoperatively; the third, as HB II preoperatively and as HB II postoperatively; and the fourth, as HB II preoperatively and as HB I postoperatively. Fourteen patients had no complications postoperatively. Of these, 6 had had a cranial nerve lesion before surgery. The period from the onset of ophthalmological dysfunction to surgery was 1 month for 5, 2 months for 1, 3 months for 2, 6 months for 1, 12 months for 1, 18 months for 1, 24 months for 1, 36 months for 1, and 72 months for 1 of the 14 patients. In 13 patients, ophthalmological functions were restored completely in the late postoperative period. The duration of ophthalmological and other neurological manifestations of the disease in patients with postoperative complications was 9 months for 1, 12 months for 2, 18 months for 1, 24 months for 2, and 180 months for 1 of the 7 patients. Of the three patients with no preoperative ophthalmological abnormalities, one patient had partial CN III palsy, and two patients, partial CN IV palsy, after surgery. All the three patients showed regression of both pain syndrome and trigeminal neuralgia. Of the nine patients who had undergone a total or near total tumor removal, 6 exhibited a complete recovery of ophthalmological functions. A partial tumor removal in a patient with a preoperative mild horizontal nystagmus also resulted in a complete recovery of ophthalmological functions. Of the 11 who had undergone a subtotal tumor removal, 5 exhibited a complete recovery of ophthalmological functions. The best surgery outcomes regarding recovery of ophthalmological functions were achieved in the patients with preoperative mild adhesion of the cyst capsule to cranial nerves who had undergone a total tumor removal. It is of note that 13 patients (61.9%) did not have symptoms of CN III, CN IV, CN V, CN VI, and/or CN VII lesions preoperatively, in spite of preoperative MRI and intraoperative evidence of the involvement of at least one of these nerves. Moderate-to-severe adhesions between the cyst capsule and nerve roots and/or brainstem were verified intraoperatively in 18/21 (85.7%) patients, and no adhesion was found in 3/21 (14.3%) patients. Table 2 demonstrates the frequency and severity of adhesions between the capsule of the epidermoid cyst and nerve roots and/or brainstem. There were 4 (22.2%) cases of severe adhesions and 1 (5.6%) case of mild adhesions for the epidermoids located at the midline of the floor of the fourth ventricle (Table 2). In addition, there were 8 (44.4%) cases of severe adhesions between the capsule of the epidermoid cyst and both nerve roots and brainstem, 4 (22.2%) cases of severe adhesions between the capsule and nerve roots only, and 1 (5.6%) case of severe adhesions between the capsule and CN only for the epidermoids located at the brainstem. The three patients with no capsule adhesion had a complete neurological and ophthalmological recovery in the late postoperative period.
The extent of epidermoid cyst removal for the cases of brainstem involvement was limited by at least a fifty-percent difference between preoperative and intraoperative somatosensory and brainstem evoked potentials. Eight of the 13 patients with no preoperative neurologic deficiency exhibited no loss of function of CN III, CN IV, CN V, CN VI, CN VII and/or brainstem in the postoperative period, in spite of moderate adhesions between the capsule and nerve roots. Out of the 21 patients, 13 (61.9%) did exhibit loss of function of CN III, CN IV, CN V, CN VI, CN VII and/or brainstem (isolated or combined cranial nerve palsy) in the postoperative period due to severe adhesions between the capsule and cranial nerves as well as efforts to perform radical tumor excision. Discussion Although there is no general consensus regarding the surgical strategy for patients with a skull-base epidermoid cyst, microneurosurgical removal of these cysts and the use of intraoperative monitoring and endoscopic equipment are believed to be essential and relevant [10, 11, 17]. Our strategy of as total as possible epidermoid removal in the presence of severe adhesions between the capsule and cranial nerve(s) and/or brainstem is in agreement with those of others [5, 6, 7, 9], but this strategy did not enable the achievement of total tumor removal in some cases. In addition, it is most likely that is was the efforts to perform radical capsule dissection that caused early postoperative neurologic loss in some of these patients. However, our data are in some disagreement with those of some previous authors [11, 12, 16], and, in the current study, the best functional treatment outcomes for the late postoperative period were observed after total or near total tumor removal. Most of the authors have noted that patients with skull-base epidermoid cysts with cranial nerve involvement were characterized by a disproportion between, on the one hand, rather substantial cranial nerve compression, dislocation and distortion and, on the other hand, minor neurological symptoms. It is likely to be caused by prolonged and slow growth of the tumor and adaptation of brain structures. Consequently, even the most careful release and dissection of cranial nerve(s) from the capsule under intraoperative monitoring and under the highest available microscope/endoscope magnification cannot guarantee complete functional preservation [1, 2, 3]. In addition, in the current study, there were cases in which total tumor removal with the dissection of capsule fragments from cranial nerves or brainstem did not result in cranial nerve dysfunction, and sometimes recovery of impaired cranial nerve functions was seen even after the dissection of these nerves from the capsule. This is also in agreement with findings of others. An improvement of cranial nerve function has been reported also after partial dissection of the epidermoid capsule [4-10]. Rehmanet and colleagues (2017) [6], Altschuler and colleagues [9] (1990), and Lynchet et al (2014) [10] reported that, in a number of cases, cranial nerve functions deteriorated early after partial or total dissection of the capsule, but neurological and ophthalmological status improved and recovered in the later postoperative period. Although the relationship between the degree of traction of neural structures and intraoperative monitoring data is important technically, we cannot identify the statistical relationship between the data of intraoperative CN monitoring and functional outcomes in the current material. However, as per our experience, it is these manipulations that should be performed under maximum microscope magnification (24х), meticulously and in close cooperation with a neurophysiologist. Given the epidemiology of these neoplasms, there is a need for further research and accumulation of multicenter data on radicality of surgery (taking into account the prevention of aseptic meningitis, cyst recurrence, intraoperative monitoring criteria, and the potential for rehabilitation of impaired cranial nerve functions) [10, 11, 17] for improved clarification of treatment strategy and obtaining statistically significant results. Therefore, total removal of complex skull-base epidermoid cysts extending into several cranial compartments can be performed through the simultaneous use of combined or two-port approaches. The dissection of the capsule from cranial nerves and/or brainstem should be performed under the highest available microscope/endoscope magnification and with intraoperative cranial nerve monitoring. In addition, the radicality of dissection depends on the degree of adhesion between the cyst capsule and surrounding neurovascular structures as well as on the potential for safe surgical manipulations on these structures. We did not observe a statistically significant relationship between the recovery of cranial nerve functions and the duration from the onset of symptoms to surgery. We found that endoscopic-assisted radical removal of the epidermoid cyst under the highest available microscope magnification with intraoperative cranial nerve monitoring was safe and effective for preserving cranial nerve functions in the late time points after surgery. References 1.You E, Bokhari R, Sirhan D. Split-Pons Syndrome by Epidermoid Cyst: A Case Report and Review of the Literature. World Neurosurg. 2019 Nov;131:275-280.e1. 2.Podeur P, Okhremchuk I, Morvan JB, Vatin L, Rivière D, de Faria A, et al. [Multiple intracranial epidermoid cysts: Case report]. Rev Laryngol Otol Rhinol (Bord). 2015;136(4):159-62. French. 3.Berger MS, Wilson CB. Epidermoid cysts of the posterior fossa. J Neurosurg. 1985 Feb;62(2):214-9. 4.Zhou F, Yang Z, Zhu W, Chen L, Song J, Quan K, et al. Epidermoid cysts of the cavernous sinus: clinical features, surgical outcomes, and literature review. Neurosurg. 2018 Oct;129(4):973-83. 5.Yaşargil MG, Abernathey CD, Sarioglu AC. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989 Apr;24(4):561-7. 6.Rehman L, Bokhari I, Siddiqi SU, Bagga V, Hussain MM. Intracranial Epidermoid Lesions: our Experience of 38 Cases. Turk Neurosurg. 2017 Oct 2. 7.Gelabert-González M. Intracranial epidermoid and dermoid cysts. Rev Neurol. 1998 Nov;27(159):777-82. 8.Caldarelli M, Massimi L, Kondageski C, Di Rocco C. Intracranial midline dermoid and epidermoid cysts in children. J Neurosurg. 2004 May;100(5 Suppl Pediatrics):473-80. 9.Altschuler EM, Jungreis CA, Sekhar LN, Jannetta PJ, Sheptak PE. Operative treatment of intracranial epidermoid cysts and cholesterol granulomas: report of 21 cases. Neurosurgery. 1990 Apr;26(4):606-13; discussion 614. 10.Lynch JC, Aversa A, Pereira C, Nogueira J, Gonçalves M, Lopes H. Surgical strategy for intracranial dermoid and epidermoid tumors: An experience with 33 Patients. Surg Neurol Int. 2014 Nov 28;5:163. 11.Shear BM, Jin L, Zhang Y, David WB, Fomchenko EI, Erson-Omay EZ, et al. Extent of resection of epidermoid tumors and risk of recurrence: case report and meta-analysis. J Neurosurgery. 2019 Jul 5;1-11. 12.Moreno-Jimenez S, Tena-Suck ML, Collado-Ortiz MA, Castillejos-López M, Alvarado-Moreno D, Holland MK. Intracranial epidermoid cyst in a single Mexican institution, experience of over 16-years. Patología. 2012;50(3):182-189. 13.Ghartimagar D, Shrestha MK, Ghosh A. Recurrence of ruptured intracranial epidermoid cyst - A rare case report and presentation. Int J Surg Case Rep. 2020;76:310-314. 14.Baldia M, Gandham E, Prabhu K. Clinico-Surgical Outcomes of Giant Intracranial Epidermoids: Gross Total Resection vs Subtotal Resection. Which is Better? Turk Neurosurg. 2021;31(4):574-581. 15.Tamura K, Aoyagi M, Wakimoto H, Tamaki M, Yamamoto K, Yamamoto M, Ohno K. Malignant transformation eight years after removal of a benign epidermoid cyst: a case report. J Neurooncol. 2006 Aug;79(1):67-72. 16.Lopes M, Capelle L, Duffau H, Kujas M, Sichez J.-P, Van Effenterre R, et al. Surgery of intracranial epidermoid cysts. Report of 44 patients and review of the literature. Neurochirurgie. 2002 Feb;48(1):5-13. 17.Hu Z, Guan F, Kang T, Huang H, Dai B, Zhu G, et al. Whole Course Neuroendoscopic Resection of Cerebellopontine Angle Epidermoid Cysts. J Neurol Surg A Cent Eur Neurosurg. 2016 Sep;77(5):381-8. 18.Abramson RC, Morawetz RB, Schlitt M. Multiple complications from an intracranial epidermoid cyst: case report and literature review. Neurosurgery. 1989 Apr;24(4):574-8. 19.Becker WJ, Watters GV, de Chadarevian JP, Vanasse M. Recurrent aseptic meningitis secondary to intracranial epidermoids. Can J Neuro Sci.1984 Aug;11(3):387-9. 20.Achard JM, Lallement PY, Veyssier P. Recurrent aseptic meningitis secondary to intracranial epidermoid cyst and Mollaret's meningitis: two distinct entities or a single disease? A case report and a nosologic discussion. Am J Med. 1990 Dec;89(6):807-10. 21.Pojskic M, Arnautovic KI. Microsurgical Resection of the Epidermoid Tumor in the Cerebellopontine Angle. J Neurol Surg B Skull Base. 2019 Jun;80(Suppl 3):S327-S328. DOI: 10.1055/s-0038-1677499.Epub 2019 Jan 31. 22.Burnham JM, Lewis K. Intracranial Extension of an Orbital Epidermoid Cyst. Ophthalmic Plast Reconstr Surg. Nov/Dec 2016;32(6):e135-e136. 23.Lane CM, Ehrlich WW, Wright JE. Orbital dermoid cyst. Eye (Lond). 1987;1 (Pt 4):504-11. 24.Mandal SK, Mandal A, Bandyopadhya A. Post Surgical Giant Epidermal Inclusion Cyst of the Lid and Orbit - A Rare Case. J Clin Diagn Res. 2015 Sep;9(9):ND01-3. 25.Miyagi A, Maeda K, Sugawara T. [Intraorbital conjunctival cyst after a penetrating orbital injury: a case report]. No Shinkei Geka.1996 Jul;24(7):649-53. Japanese. 26.König B, Vána V, Vojácek K. [Intracranial epidermoids. Characteristic observations concerning epidermoids in the region of the optic chiasm]. Klin Monbl Augenheilkd.1966;148(2):190-202. German. Conflict of interest: The authors state that there is no actual or potential conflict of interest (financial, personal, professional and other interests) that could affect the opinion on the subject or materials described and discussed in this manuscript. Disclaimer: The opinions expressed in the presented article are the authors' own, and not the official positions of the institution.
|