J.ophthalmol.(Ukraine).2021;6:41-47.
|
http://doi.org/10.31288/oftalmolzh202164147 Received: 10 September 2021; Published on-line: 21 December 2021 Hypotensive effect of endotrabeculectomy as a function of the stage of glaucoma and preoperative intraocular pressure in patients with primary open-angle glaucoma I. Ia. Novytskyy, O. V. Levytska Danylo Halytsky Lviv National Medical University E-mail: Inovytskyy@gmail.com TO CITE THIS ARTICLE: Novytskyy IIa, Levytska OV. Hypotensive effect of endotrabeculectomy as a function of the stage of glaucoma and preoperative intraocular pressure in patients with primary open-angle glaucoma. J.ophthalmol.(Ukraine). 2021;6:41-47. http://doi.org/10.31288/oftalmolzh202164147
Background: In recent years, minimally invasive glaucoma surgery (MIGS) (particularly, ab interno MIGS) has been actively developed. Various studies have focused on the efficacy of these procedures. Purpose: To assess intraocular pressure (IOP) reduction from baseline after controlled endotrabeculectomy (ETE) in patients with different stages of primary angle glaucoma (POAG) and preoperative IOP levels. Material and Methods: Eighty-eight patients (88 eyes) that received controlled endotrabeculectomy for POAG were included in the study. The 88 patients were divided into two groups based on the stage of glaucoma. In addition, these patients were divided into two other groups based on the preoperative IOP. IOP readings were obtained with a Maklakoff tonometer preoperatively and on day 7 and 1, 3, 6, and 9 and 12 months after surgery. The numbers of hypotensive medications used and visual acuities at the above time points were also noted. Results: The difference between preoperative IOP and postoperative IOP was significant for all groups until month 12 (p < 0.05), irrespective of the stage of glaucoma and the preoperative IOP. The intragroup difference between preoperative and postoperative numbers of hypotensive medications was significant (p < 0.05) for group 1 and group 3 until month 12, for group 2 until month 9, and for group 4 until month 6. The difference at subsequent time points (month 9 and month 12) was not significant. Conclusion: Controlled endotrabeculectomy can be recommended for patients with stage 1 of stage 2 of glaucoma and/or a baseline IOP of 24 mmHg or lower. In addition, it can be recommended for patients with a baseline IOP of 25 mmHg or higher and patients with stage 3 of stage 4 of glaucoma, but in these cases, one should take into account a high probability of requirement of topical hypotensive medications in the postoperative period. Keywords: primary open-angle glaucoma, endotrabeculectomy, intraocular pressure, topical hypotensive medication
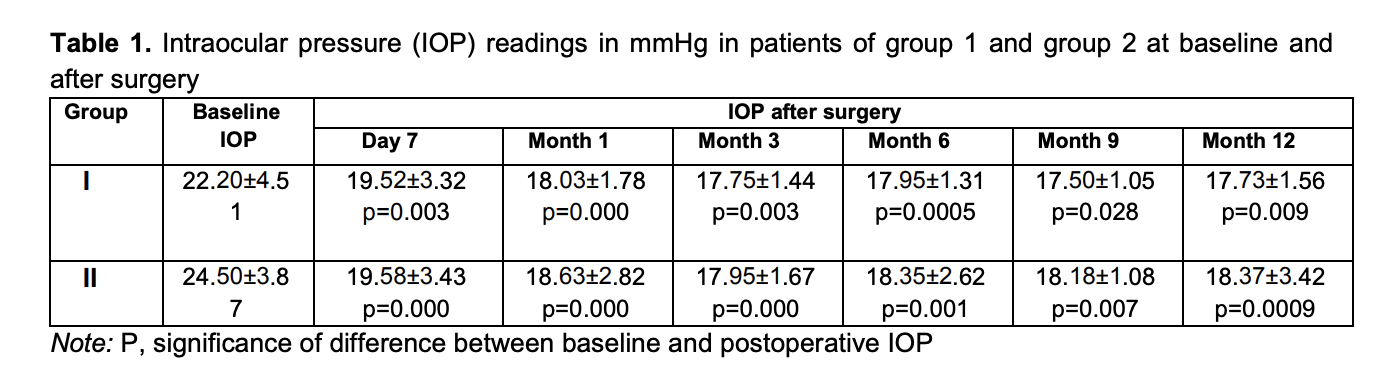
Introduction Primary open-angle glaucoma is a complex and multifactorial ocular disorder and a serious medical and social concern. Glaucoma is a major global cause of blindness and is found in countries with various socioeconomic statuses. The disorder can lead to irreversible loss of peripheral and central vision [1]. In Ukraine, glaucoma accounts for 40.2% of all adults with certified visual disability [2]. In 1996, the number of people (aged 40-80 years) with glaucoma worldwide was estimated to be 40 million, increasing to 112 million in 2040. An increase in the number of persons affected by glaucoma (especially, in the developed countries) is associated with aging, which points to an important role of innovations in the onset and development of the disorder [3]. Primary open-angle glaucoma is a polyetiologic disease. Age, hereditary factor, some somatic disorders (hypertensive disease, cardiovascular atherosclerosis, etc.) are believed to be risk factors for glaucoma. However, elevated IOP is a major unfavorable factor [4]. Therefore, ocular hypotensive treatment prevents or delays the onset of visual function loss and is still the mainstay of treatment for glaucoma. Glaucoma surgery is used if the conservative treatment cannot lower the IOP adequately. The creation of new outflow channels is achieved by glaucoma filtration procedures (particularly, sinus trabeculectomy), the most commonly used surgical procedures for glaucoma treatment. They are popular because are easy to perform and effective. Despite these benefits, complications (like choroidal detachment, inflammation, and the development of cataract) are common in these procedures, leading to a further reduction in vision [5]. In recent years, minimally invasive glaucoma surgery (MIGS) (particularly, ab interno MIGS) has been actively developed 6]. Various studies have focused on the efficacy of these procedures. The purpose of the study was to assess IOP reduction after controlled endotrabeculectomy (ETE) in patients with different stages of POAG and preoperative IOP levels. Material and Methods Eighty-eight patients (88 eyes) that received controlled endotrabeculectomy for POAG were included in the study. Informed consent for treatment was signed by all patients of the study. For the purpose of the analysis of IOP reduction based on the stage of glaucoma, the 88 patients were divided into two groups, group 1 of 40 patients (40 eyes) with stage 1 glaucoma or stage 2 glaucoma, and group 2 of 48 patients (48 eyes) with stage 3 glaucoma or stage 4 glaucoma. For the purpose of the analysis of IOP reduction based on the preoperative IOP, the 88 patients were divided into another two groups, group 3 of 58 patients (58 eyes) with an IOP ≤ 24 mmHg, and group 4 of 30 patients (30 eyes) with an IOP > 25 mmHg. IOP readings were obtained with a Maklakoff tonometer preoperatively and on day 7 and 1, 3, 6, and 9 and 12 months after surgery. The number of hypotensive medications used at the above time points was also noted. Any combination glaucoma medication was taken into account based on the number of individual components in this medication. Visual acuity was also assessed at the above time points. Surgery technique Epibulbar anesthesia with proparacaine hydrochloride (Alcain; Alcon-Couvreur, Puurs, Belgium) was administered. A 1.2-mm anterior chamber paracenthesis was performed at 3 and 10 o’clock, 1% lidocaine was injected into the anterior chamber, and the anterior chamber was filled with viscoelastic. A forceps was used to remove two strings of the trabecular meshwork (one of 2 clock hours in one quadrant, and another of 3 clock hours in another quadrant, 110 to 120 degrees totally) under gonioscopic control. The viscoelastic was washed out from the anterior chamber using an irrigation-aspiration system, and the paracentesis wounds were hydrated. Statistical analyses were conducted using Statistica 12.0 (StatSoft, Tulsa, OK, USA) software. The Mann-Whitney U test was used for comparisons between groups at particular time points. The Wilcoxon t test was used to compare baseline and post-treatment values for the IOP and the number of hypotensive medications used. Results Mean IOP values in group 1 and group 2 were 22.20 ± 4.51 mmHg and 24.50 ± 3.87 mmHg, respectively, preoperatively; 19.52±3.32 mmHg and 19.58±3.43 mmHg, respectively, at day 7; 18.03 ± 1.78 mmHg and 18.63 ± 2.82 mmHg, respectively, at 1 month; 17.75 ± 1.44 mmHg and 17.95 ± 1.67 mmHg, respectively, at 3 months; 17.95 ± 1.31 mmHg and 18.35 ± 2.62 mmHg, respectively, at 6 months; 18.18 ± 1.08 mmHg and 18.18 ± 1.08 mmHg, respectively, at 9 months; and 17.73 ± 1.56 mmHg and 18.37 ± 3.42 mmHg, respectively, at 12 months (Table 1).
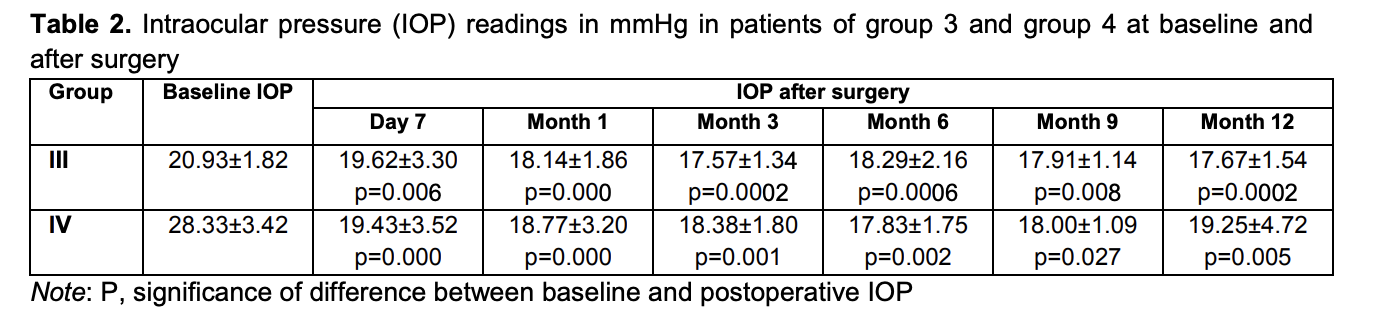
Mean IOP values in group 3 and group 4 were 20.93 ± 1.82 mmHg and 28.33 ± 3.42 mmHg, respectively, preoperatively; 19.62 ± 3.30 mmHg and 19.43 ± 3.52 mmHg, respectively, at day 7; 18.14 ± 1.86 mmHg and 18.77 ± 3.20 mmHg, respectively, at 1 month; 17.57 ± 1.34 mmHg and 18.38 ± 1.80 mmHg, respectively, at 3 months; 18.29 ± 2.16 mmHg and 17.83 ± 1.75 mmHg, respectively, at 6 months; 17.91 ± 1.14 mmHg and 18.00 ± 1.09 mmHg, respectively, at 9 months; and 17.67 ± 1.54 mmHg and 19.25 ± 4.72 mmHg, respectively, at 12 months (Table 2).
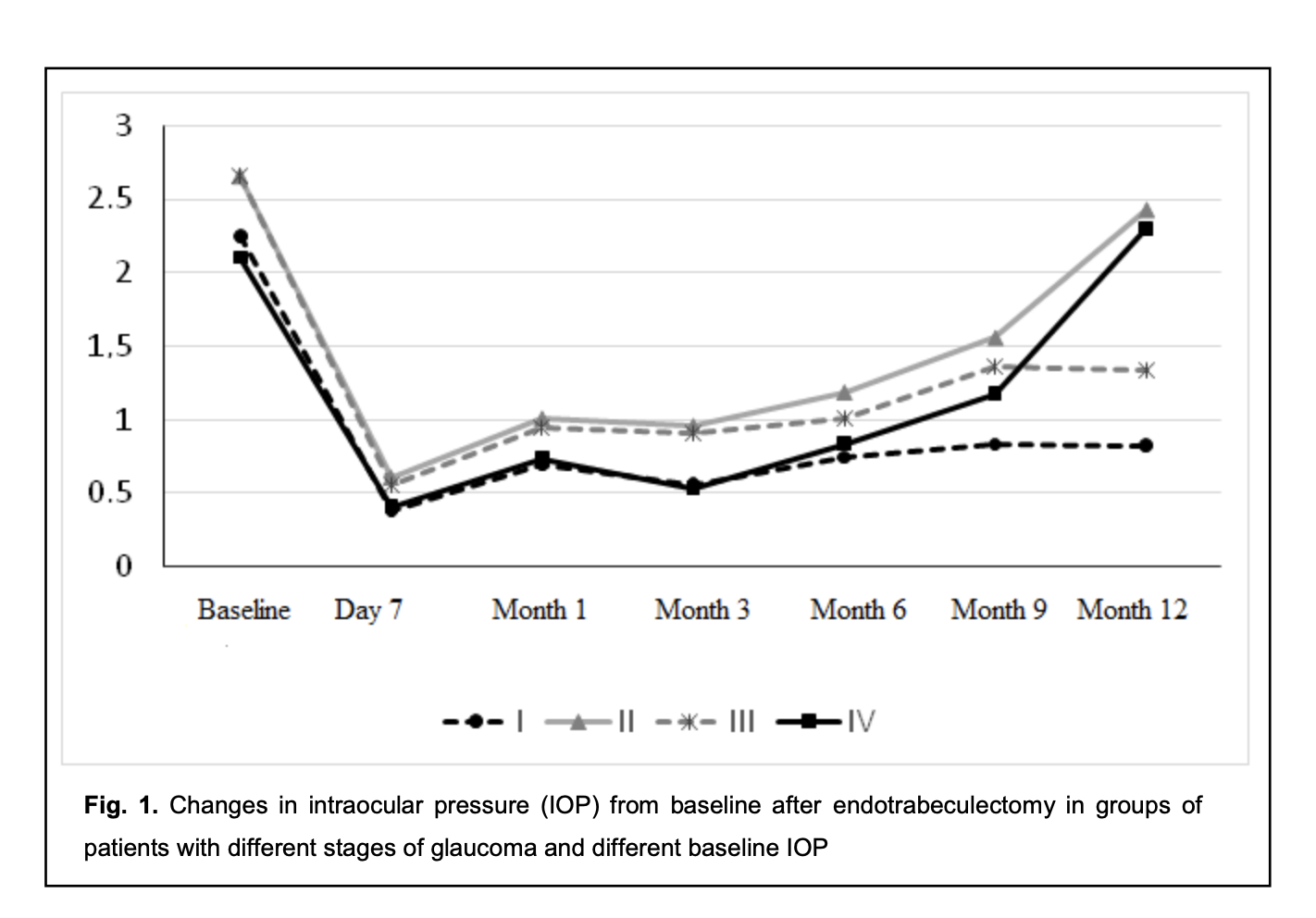
The difference between preoperative IOP and postoperative IOP was significant for all groups until month 12 (p < 0.05), irrespective of the stage of glaucoma and the preoperative IOP (Fig. 1).
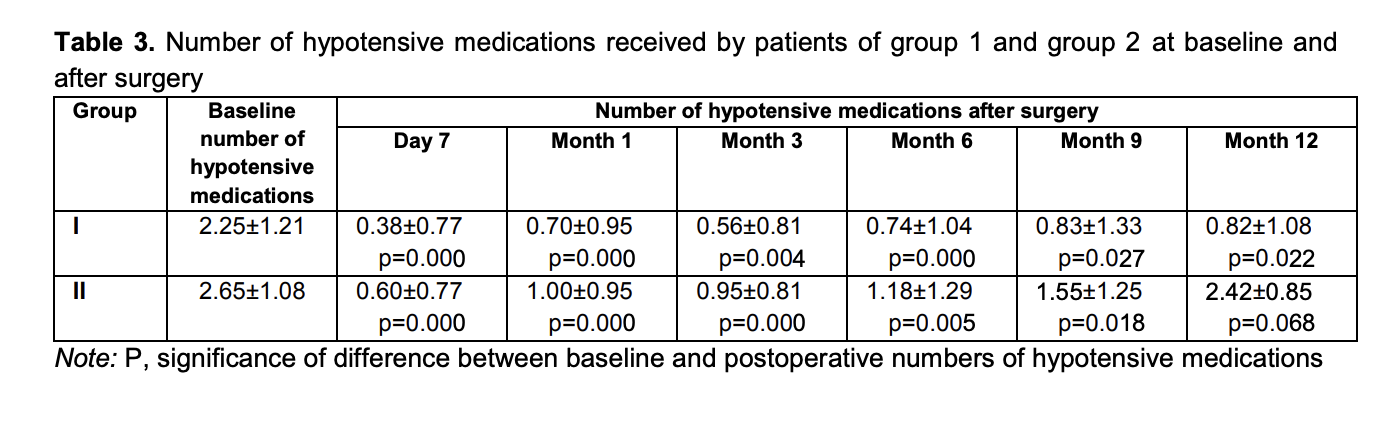
Mean numbers of hypotensive medications used for group 1 and group 2 were 2.25 ± 1.21 and 2.65 ± 1.08, respectively, preoperatively; 0.38 ± 0.77 and 0.60 ± 0.77, respectively, at day 7; 0.70 ± 0.95 and 1.00 ± 0.95, respectively, at month 1; 0.56 ± 0.81 and 0.95 ± 0.81, respectively, at month 3; 0.74 ± 1.04 and 1.18 ± 1.29, respectively, at month 6; 0.83 ± 1.33 and 1.55 ± 1.25, respectively, at month 9; and 0.82 ± 1.08 and 2.42 ± 0.85, respectively, at month 12 (Table 3).
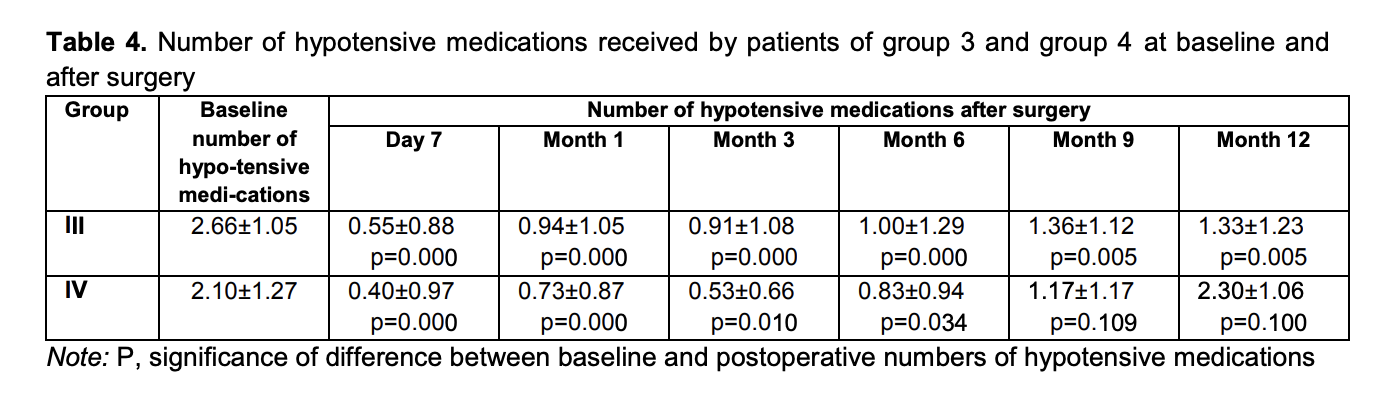
Mean numbers of hypotensive medications used for group 3 and group 4 were 2.66 ± 1.05 and 2.10 ± 1.27, respectively, preoperatively; 0.55 ± 0.88 and 0.40 ± 0.97, respectively, at day 7; 0.94 ± 1.05 and 0.73 ± 0.87, respectively, at month 1; 0.91 ± 1.08 and 0.53 ± 0.66, respectively, at month 3; 1.00 ± 1.29 and 0.83 ± 0.94, respectively, at month 6; 1.36 ± 1.12 and 1.17 ± 1.17, respectively, at month 9; and 1.33 ± 1.23 and 2.30 ± 1.06, respectively, at month 12 (Table 4).
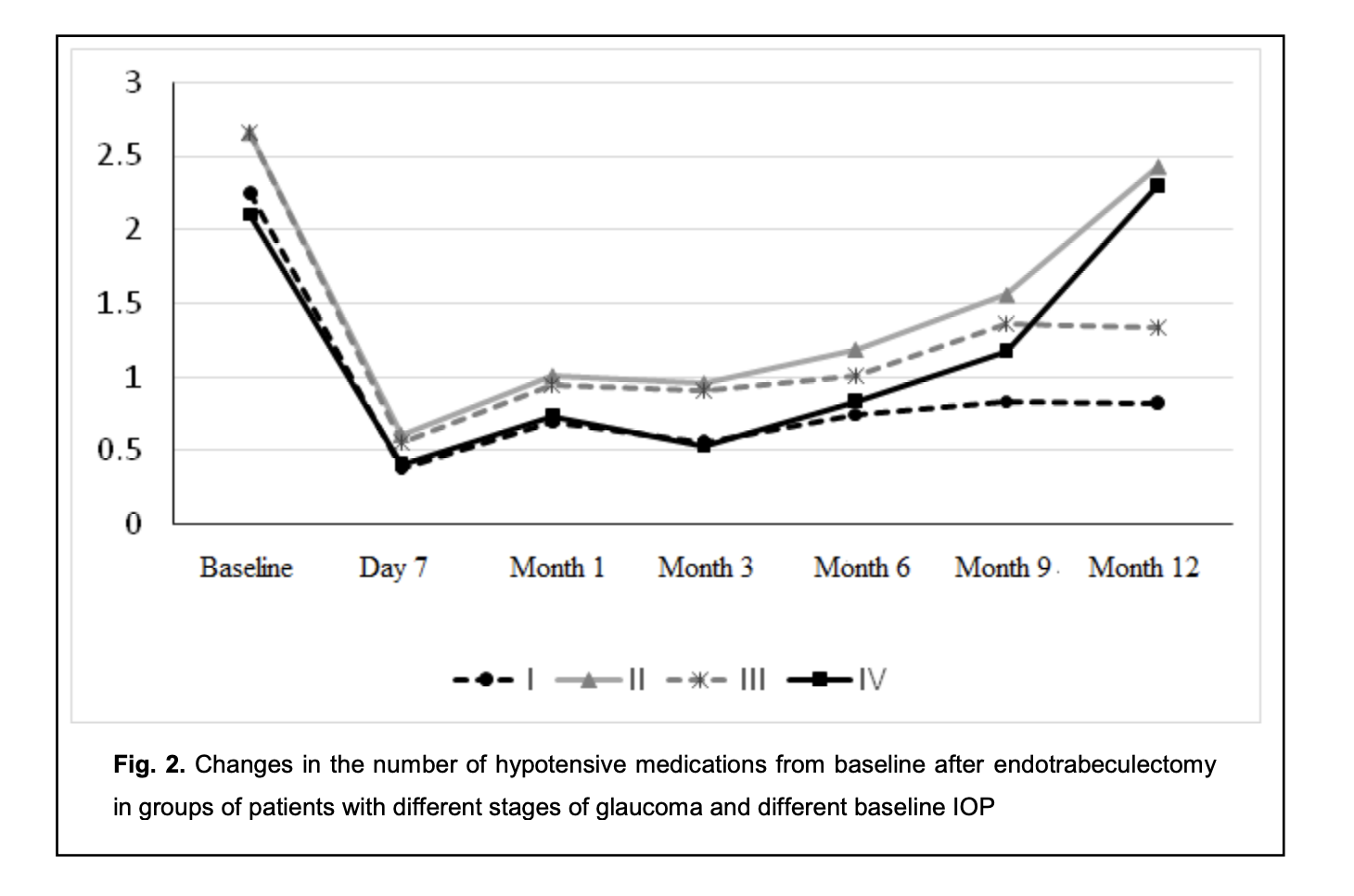
The intragroup difference between preoperative and postoperative numbers of hypotensive medications was significant (p < 0.05) for group 1 and group 3 until month 12, for group 2 until month 9, and for group 4 until month 6. The difference at subsequent time points (month 9 and month 12) was not significant (p > 0.05) (Fig. 2).
The results obtained indicate that there was a significant decrease in the number of hypotensive medications used by patients with stage 1 or stage 2 glaucoma and in those with preoperative IOP of 24 mmHg or lower, by month 12 postoperatively. However, the difference between preoperative number of hypotensive medications and postoperative number of hypotensive medications used by patients with stage 3 or stage 4 glaucoma was not significant after month 9. In addition, the difference between preoperative number of hypotensive medications and postoperative number of hypotensive medications used by patients with preoperative IOP of 25 mmHg or more was not significant as early as after month 6. Moreover, the preoperative number of topical hypotensive medications was practically the same for all the four groups. No significant difference (p > 0.05) in IOP was observed between group 1 and group 2 at month 6 and month 12. However, when taking in account the number of topical hypotensive medications used by patients of group 1 and group 2, there was a significant difference for month 12 (compared to patients of group 1, patients of group 2 had to receive more hypotensive drops in order to receive the same IOP). No significant difference (p > 0.05) in IOP was observed between group 3 and group 4 at any time point. The number of topical hypotensive medications used by patients of group 3 was practically the same as that for patients of group 4 at day 7 and at 1, 3 and 6 months, with no significant difference (p > 0.05), but the number of topical hypotensive medications used was greater for group 4 than for group 3 at 9 months and 12 months. A portion of patients (65 of 88 patients; 65 eyes) received not only a controlled ETE procedure, but also phacoemulsification with intraocular lens (IOL) implantation. Table 5 presents visual acuities for these patients. In these patients, there was a significant (p < 0.05) increase in visual acuity after surgery, likely due to cataract phacoemulsification. Others (23 of 88 patients; 23 eyes) received only a controlled ETE procedure. Of these 23 patients, 12 had an IOL. Table 5 presents visual acuities for these patients. As there was no significant change in visual acuity after surgery in these 12 patients, an ETE procedure had practically no effect on visual acuity. Hyphema was the most common postoperative complication, and was seen in 14 of 88 patients (15.9%). Although choroidal detachment, impaired circulation in the retina and/or optic nerve, and hypotony are typical for glaucoma filtration procedures, none of these was observed. The results obtained indicate that ab interno trabeculectomy is effective in patients with primary open-angle glaucoma irrespective of the stage of glaucoma or the preoperative IOP. However, it should be noted that the amount of hypotensive effect provided by this procedure depends from the stage of glaucoma and preoperative IOP, and patients with stage 3 or stage 4 glaucoma and/or preoperative IOP of 25 mmHg or more had to receive a greater number of hypotensive drops than patients with stage 1 or stage 2 glaucoma and/or preoperative IOP of 24 mmHg or less. These data should be taken into account when selecting the surgical strategy in patients that require not only IOP reduction, but also a reduction in the number of postoperative instillations of hypotensive drops (e.g., when there is intolerability to topical hypotensive medications, or instillation is impossible due to the patient’s loss of fine manual skills). Discussion There are few studies comparing the efficacy of ab interno trabeculectomy in patients with different stages of glaucoma and different levels of preoperative IOP. An advantage of the current study is that it assesses the hypotensive effect of endotrabeculectomy as a function of the above factors, which would enable an improved outlining of indications for MIGS. A review of Godfrey and colleagues [7] concentrated on three of the more commonly performed canal procedures: trabeculotomy ab interno (Trabectome), Canaloplasty, and trabeculotomy ab externo. They found that trabeculotomy ab interno performed with the Trabectome lowered IOP almost 40% by 12 months with minimal complications, whereas the current study found that the controlled ETE lowered IOP by 23% by 12 months (from 23.45 ± 4.30 mmHg preoperatively to 18.00 ± 2.45 mmHg at 12 months. In our study, there were minimal complications, which is in agreement with findings of Godfrey and colleagues [7]. Grover and colleagues [8] introduced a minimally invasive, ab interno approach to a circumferential 360-degree trabeculotomy and reported the preliminary results. They found that in patients with POAG, the IOP decreased by 7.7 ± 6.2 mm Hg at 6 months, which is close to our findings (the IOP decreased by 5.32 ± 2.29 mm Hg at 6 months). The guidelines [9] recommend a 20%-30% reduction in IOP from baseline as a criterion of efficacy for glaucoma surgery, which was achieved in the current study. When assessing the efficacy of the controlled endotrabeculectomy as a glaucoma surgery depending on the baseline IOP, the efficacy was 15.6% for the group with a baseline Maklakoff IOP of 24 mmHg or lower, versus 32.05% the group with a baseline Maklakoff IOP of 25 mmHg or higher, although with a target Maklakoff IOP lower than 20 mmHg, the efficacy in the former group would be the same as in the latter group (mean Maklakoff IOP, 18-19 mmHg). However, when assessing the efficacy of the controlled endotrabeculectomy as a glaucoma surgery depending on the stage of glaucoma, there was no substantial difference between the efficacy for patients with patients with stage 1 or stage 2 glaucoma (20.18%) and patients with stage 3 or stage 4 glaucoma (25.02%). The number of topical hypotensive medications used is an important criterion of the efficacy of glaucoma surgery. In the current study, the number of topical hypotensive medications used by patients with a baseline Maklakoff IOP of 25 mmHg or higher and with stage 3 or stage 4 glaucoma at month 12 after glaucoma surgery was significantly higher than the number of topical hypotensive medications used by patients with a baseline Maklakoff IOP of 24 mmHg or lower and with stage 1 or stage 2 glaucoma (although the preoperative number of topical hypotensive medications used by the former patients was the same as that used by the latter patients), with no significant difference in the postoperative IOP. The results obtained indicate that the preoperative IOP, stage of glaucoma, and number of topical hypotensive medications used are not contraindications for controlled endotrabeculectomy. With regard to the the assessment of the hypotensive effect of surgery, we may conclude that the probability of absolute success of surgery (defined as achieving the target IOP without glaucoma medications) was higher in patients with a baseline Maklakoff IOP of 24 mmHg or lower and with stage 1 or stage 2 glaucoma than in patients with a baseline Maklakoff IOP of 25 mmHg or higher and with stage 3 or stage 4 glaucoma, but the probability of qualified success of surgery (defined as achieving the target IOP with the additional use of glaucoma medications) was higher in the latter patients. The results obtained are important also because when selecting the most suitable surgical procedure, it is necessary to take into account the number of topical hypotensive medications predicted for the postoperative period. Conclusion First, the controlled endotrabeculectomy was found to induce an apparent hypotensive effect in patients with primary open-angle glaucoma of any stage of glaucoma and different preoperative IOP levels. Second, in order to achieve the target IOP by month 12 after surgery, patients of group 2 (i.e., patients with stage 3 or stage 4 glaucoma) had to receive a significantly greater number of topical hypotensive medications than patients of group 1 (i.e., patients with stage 1 or stage 2 glaucoma). Third, in order to achieve the target IOP at month 9 and month 12 after surgery, patients of group 4 (i.e., patients with a baseline Maklakoff IOP of 25 mmHg or higher) had to receive a significantly greater number of topical hypotensive medications than patients of group 3 (i.e., patients with a baseline Maklakoff IOP of 24 mmHg or lower).
Finally, it is reasonable to consider alternative types of glaucoma surgery in patients with a baseline Maklakoff IOP of 25 mmHg or higher, or patients with stage 3 or stage 4 glaucoma, when there is intolerability to topical hypotensive medications, or instillation is impossible due to patient’s loss of fine manual skills. References 1.Vitovska OP, Alifanofa TA, Poveshchenko YuL. [Epidemiological aspects of primary disability caused by glaucoma in Ukraine]. Oftalmologiia. 2016;2:16-25. Russian. 2.Ukrainian Medical and Dental Academy. [Glaucomas. Optic nerve disorders]. 2019. Ukrainian. 3.Novytskyy IIa. [Current surgery for primary open-angle glaucoma. A shift towards minimally invasive surgery]. Lviv:Litopys;2018. p.106-119. Ukrainain. 4.Musch DC, Gillespie BW, Niziol LM, et al. Factors Associated with Intraocular Pressure before and during 9 Years of Treatment in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2008 Jun;115(6):927-33. doi: 10.1016/j.ophtha.2007.08.010. 5.Zavgorodnia NG, Sarzhevskyi AS. [Results of sinus trabeculectomy and iridectomy for the management of glaucoma combined with cataract]. Zaporozhskyi medychnyi zhurnal. 2015;89(2):70-3. Ukrainian. 6.Novytskyy M. [Pathogenetic grounds for ab interno endotrabeculectomy for primary open-angle glaucoma]. Arkhiv oftalmologii Ukrainy. 2014;59:54-9. Ukrainian. 7.Godfrey DG, Fellman RL, Neelakantan A. Canal surgery in adult glaucomas. Curr Opin Ophthalmol. 2009 Mar;20(2):116-21. doi: 10.1097/ICU.0b013e32831eef65. 8.Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014 Apr;121(4):855-61. doi: 10.1016/j.ophtha.2013.11.001. 9.European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Publicomm: Savona, Italy. 2017
|