J.ophthalmol.(Ukraine).2015;5:3-6.
|
https://doi.org/10.31288/oftalmolzh2015536 Importance of clinical and cytological factors for prediction of efficacy of treatment in persistent corneal epithelial defects and ulcers from different causes L.F. Troichenko1, Cand. Sc. (Med), G.I. Drozhzhina1, Dr. Sc. (Med), Prof., V.V. Vit 1, Dr. Sc. (Med), Prof., V.V. Filonenko2, Dr. Sc. (Biol), Prof., A.I. Khoruzhenko2, Cand. Sc. (Biol), Sen. Res. 1 Filatov Eye Disease and Tissue Therapy Institute, the NAMS of Ukraine, Odessa 2 Molecular Biology and Genetics Institute, the NAS, Ukraine E-mail: lf2008@ukr.net
Background: Characteristics of persistent corneal epithelial defects (PCEDs) of neurotrophic origin are a long clinical course and abnormal epithelial regeneration; additionally, patients often have complications such as stromal thinning and corneal perforation. Purpose: To investigate the clinical and cytological factors influencing the efficacy of treatment of PCEDs. Materials and Methods: The clinical and cytological factors for prediction of efficacy of treatment in PCEDs of different causes were investigated in 51 patients. We performed general ophthalmological examinations and cytological and immunohistochemical analysis using Millicell filters and antibodies against cytokeratins (CK), “corneal” CK3 and “conjunctival” CK19. Results: The following factors were found to be of significant influence on the achievement of the outcome target (complete re-epithelialization of the corneal surface): size of corneal defect; flat margins of corneal defect; presence of basal cells in cytograms; absence of the cells expressing “corneal” CK3 and presence of the cells expressing “conjunctival” CK19 in immunohistochemistry. Univariant logistic regression was used for the quantitative assessment of the risk of failure to achieve complete re-epithelialization of corneal defects. Conclusion: Using the mathematical model proposed, one can predict the achievement of complete re-epithelialization of the corneal surface with the conservative therapy in 74.5% of the cases with the sensitivity of 72.2% and specificity of 78.8%. Keywords: persistent corneal epithelial defects, efficacy of treatment, prediction, clinical and cytological factors
Introduction Characteristics of persistent corneal epithelial defects (PCEDs) of neurotrophic origin are a long clinical course and regeneration disorders; additionally, patients often have complications such as corneal stromal thinning and corneal perforation. PCEDs are considered as a corneal degeneration caused by impairment of the corneal sensory innervation. This results in a loss of corneal sensation and reduced tear production, leading to corneal metabolic disorders, degenerative epithelial cell changes, and reduced mitotic activity in epithelial cells [1-8]. In persistent corneal defects, one may observe reparative regeneration at the expense of corneal epithelial cells or regeneration through the replacement of these defects with connective tissue without complete morphological and functional restoration [9,10]. Previously, we have shown [11] that predominance of corneal epithelial over corneal conjunctival cells and the corneal epithelial to corneal conjunctival cell ratio of 1:1 are favorable prognostic signs for the time course and quality of re-epithelialization. However, no clinical and cytological factors statistically significantly influencing corneal regeneration have been revealed. Therefore, the purpose of the present study was to investigate the clinical and cytological factors influencing the efficacy of treatment of persistent corneal epithelial defects and ulcers.
Materials and Methods The study included 51 inpatients (27 men, 24 women) with the slow (more than 10 days) neurotrophic or post-infectious corneal surface regeneration of the Cornea Pathology Department, the Filatov Institute of Eye Diseases & Tissue Therapy. All eyes were treated with conservative etiotropic and pathogenetic therapy including autologous serum instillation. To assess the clinical picture of ocular pathology, ophthalmic examination was performed involving the following: anterior biomicroscopy with investigation of the features of margins of the corneal defect; corneal fluorescein staining test to determine the size and depth of the corneal defect; determination of corneal sensation (Faulkner’s technique); tear film stability measurement (by Norn); Schirmer I test for total tear production; non-contact tonometry; fundus inspection; best corrected visual acuity; photo registration of alterations in the anterior eye. Margins of corneal defects were assessed as being flat or edematous-and-overhang. Patient age ranged from 27 to 77 years (54±14.1 years). Corneal defect size ranged from 3.0 to 6.5 (4.4±1.0) mm. Impression cytology specimens of the corneal surface were collected using Millicell®–CM 0.4 ?m (Millipore, Bedford, MA, USA) filters. Additionally, immunohistochemistry with antibodies against cytokeratins (CK), CK3 and CK19, was performed to detect corneal and conjunctival epithelia, respectively. Immunohistochemistry was performed at the Institute of Molecular Biology and Genetics, the National Academy of Sciences of Ukraine. The data base was developed using Data Manager module of Statistica 9 (StatSoft Corp.) software. Binary logistic regression was used to model the relationship between a set of independent variables and the achievement of complete epithelialization of corneal defects. Receiver-Operating-Characteristic (ROC) analysis was performed using MedCalc 9.1 software. ROC curve (sensitivity plotted against 1-specificity) was constructed. The area under the ROC curve was calculated and the optimal cut-off point on the curve was determined.
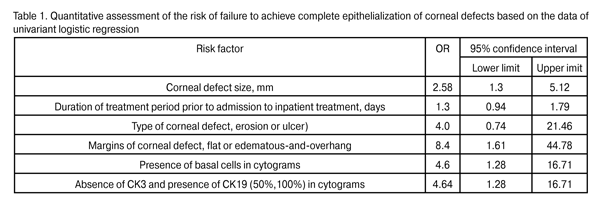
Results Patients were divided into two groups based on the clinical analysis of the quality of corneal regeneration. Group 1 involved 33 patients (33 eyes; 64.7%) whose eyes had achieved complete re-epithelialization, whereas Group 2 involved 18 patients (36.3%) whose eyes had not achieved it. In Group 1 eyes, viral (54.5%) and neurotrophic (30.3%) etiologies of primary ocular pathological process prevailed, and corneal ulcers were more frequent (22 eyes; 66.7%) than corneal erosions (11 eyes; 33.3%). In Group 2 eyes, viral, bacterial and neurotrophic etiologies of primary ocular pathological process were represented almost equally (23.5%, 23.5%, and 29.4% respectively), and corneal ulcers were much more frequent (15 eyes; 88.2%) than corneal erosions. In Group I, flat margins of corneal defects were observed in 51.5% of cases, whereas failure to achieve complete epithelialization was accompanied by edematous-and-overhang margins of corneal defects in 88.2% (?2=7.52, P = 0.006) of cases. Most of the corneal defects in Group 1 (54.5%) and Group 2 (58.8%) had moderate opacification and apparent opacification, respectively. The size of corneal defects in Group 1 (4.3±1.0 mm) differed statistically significantly from that in Group 2 (5.3±1.0 mm; P = 0.002). Decreased corneal sensation was present in 69.7% and 61.1% of Group 1 and Group 2 patients, respectively. Tear production indices were low (5.1± 2.1 mm) and did not differ significantly between the groups. In cytological impression of corneal defects in Group 2 eyes, basal cells prevailed (55.6%), whereas in that of Group 1 eyes, superficial epithelial cells and mixed cell composition (78.8%) (?2=6.18, P = 0.012) were mostly noted. Microbial inclusions were absent in eyes of both groups. Presence of CK3 and CK19 was found in 78.7% and 21.2%, respectively, of Group 1 eyes, versus 47% and 53% of Group 2 eyes (?2=6.07, P = 0.04). Univariant logistic regression (1, presence of a risk factor; 0, absence of risk factor) was used for the quantitative assessment of the risk of failure to achieve complete epithelialization of corneal defects (Table 1).
Based on the assessment of the factors potentially influencing the achievement of the clinical outcome, a subset of variables was identified that had the greatest influence on the prognosis of the achievement of complete epithelialization. These variables got the information load from the rest of the signs under investigation which were not introduced into the mathematical model. Therefore, the values of the following indices in the achievement of the clinical outcome (complete epithelialization of the corneal surface) are most important and should be considered in the prediction of the efficacy of treatment taking into account their common influence on the outcome: (1) edematous-and-overhang state of the defect margin; (2) presence of basal cells in cytograms; (3) absence of CK3 and presence of CK19 in immunohistochemistry. The three following signs were adopted as predictors as a result of step-by-step inclusion of the most valuable variables into the non-efficacy prediction model: X1 (absence of CK3 and presence of CK19 in immunohistochemistry), X2 (presence of basal cells in cytograms) and X3 (edematous-and-overhang state of the defect margin). Table 2 presents the logistic regression equation coefficients and their significance. The total value of the model was found highly significant (c2=19.28; P = 0.0002).
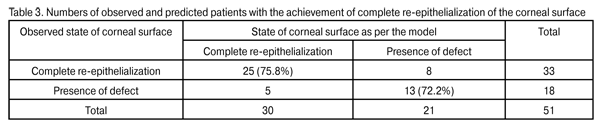
Therefore, using the coefficients presented in Table 2 one can determine the probability of failure to achieve complete epithelialization of corneal defects (alternatieve, 1-P, achievement of complete epithelialization of the corneal surface). Y=-3.361694+ Х1•1.789416+Х2•1.595375+ Х3•2.070957 odds=exp(Y); Р=odds/(odds+1) The probability of failure to achieve complete epithelialization of corneal defects (maintenance of the defect) was computed for every patient using logistic regression equation coefficients. The overall percentage of correct diagnosis was 74.51%. In patients with complete re-epithelialization of the corneal surface, the percentage of correct diagnosis was 75.8%, with 8 patients having a false positive diagnosis. In patients with a failure to complete re-epithelialization of the corneal surface, the percentage of correct diagnosis was 72.2%, with 5 eyes falsely diagnosed as having a completely re-epithelialized corneal surface (Table 3).
Here are clinical examples of patients with calculated statistical indices related to failure to complete re-epithelialization of the corneal surface. As one can see from Table 4, in patient D, all the three risk factors were present, odds ratio (OR) was 8.118, the probability of failure to remove the corneal defect was 0.89, conforming to the outcome observed. In patient B, only one risk factor (absence of CK3 and presence of CK19) was present, OR was 0.207, the probability of failure to remove the corneal defect was 0.17, conforming to the clinical outcome observed (the treatment resulted in complete epithelialization of the corneal surface).
Overall, the calculated probability of failure to achieve complete re-epithelialization of the corneal surface varied from 0.03% (with all the three risk factors being absent) to 89% (with all the three risk factors being present). ROC analysis was used to obtain the operational characteristic of the test (mathematical model) for the prediction of probability to achieve complete epithelialization of the corneal surface (Fig. 1).
Fig.1. Operational characteristic of the test (mathematical model) for the prediction of probabikity of achieving complete epithelization of the corneal surface The area under ROC curve was 0.82, and cut-off point was 0.51. For this cut-off point, the sensitivity was 72.22% (95% CI, 46.5-90.2) and specificity 83% (95% CI, 61.1-91.0). Therefore, the ROC-analysis performed showed that in patients with OR <0.51, complete epithelialization of the corneal surface is achieved with the conservative treatment used. Using the mathematical model proposed in the course of conservative treatment, one can predict the probability of achieving complete re-epithelialization of corneal defects based on the values of the predictor factors mentioned above. From the results obtained, the conclusion can be drawn that the conservative treatment should be continued in patients with OR < 0.51, whereas surgical treatment is reasonable in patients with OR > 0.51. This will lead to reduction in inpatient days for the patient, and to a more rational distribution of material resources required for treatment of patients. Conclusion 1. The following factors influence statistically significantly complete epithelialization of corneal defects in patients with PCEDs: (1) size of corneal defect, (2) flat margins of corneal defect, and (3) predominance of superficial epithelial cells and cells expressing CK3 in cytograms. 2. Such factors as the absence of edematous-and-overhang margins of corneal defect and basal cells, and the presence of cells expressing CK3 in cytograms are significant predictors of complete re-epithelialization of the corneal surface in PCEDs. 3. Using the mathematical model proposed, one can predict the achievement of complete re-epithelialization of the corneal surface with the conservative therapy in 74.5% of the cases with the sensitivity of 72.2% and specificity of 78.8%. Acknowledgment: The authors appreciate sincerely the help of E.I. Dragomiretskaia who have assisted in statistical analysis.
References 1. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003 Nov;17(8):989-95. 2. Goins KM. New Insights into the Diagnosis and Treatment of Neurotrophic Keratopathy. Ocul Surf. 2005; Apr. 3(2):96-110. 3. Madhusudan, GR, Sharma, RK, Nanda, V. Neuroparalytic keratitis: A rare manifestation of posttraumatic superior orbital fissure syndrome. Ann Plast Surg. 2004 Jul;53(1):83-5. 4. Tuominen IS Konttinen YT Vesaluoma MH. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–2549. 5. Barbaro V, Ferrari S, Fasolo A. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:926-32. 6. Yamada N, Matsuda R, Morishige N. Open clinical study of eye – drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896-900. 7. Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma treatment. Br J Ophthalmol. 2010;94:584-91. 8. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014 Mar;8:571-9. 9. Cavanagh HD, Colley AM. The molecular basis of neurotrophic keratitis. Acta Ophthalmol Suppl 1989; 192: 115–134. 10. Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of Limbal Stem Cell Deficiency by Corneal Impression Cytology. Cornea. 2003;22(6):533-8. 11. Vit VV, Drozhzhina GI, Troichenko LF, Filonenko VV, Khoruzhenko AI, Cherednik OV. [Importance of corneal epithelial to corneal conjunctival cell ratio in persistent corneal epithelial defects and ulcers of postinfectious and neuroparalytic etiology]. Oftalmol Zh. 2012;(4):7-12. Russian. |