J.ophthalmol.(Ukraine).2019;4:28-32.
|
http://doi.org/10.31288/oftalmolzh201942832 Changes in immunity characteristics in patients with small (T1) choroidal melanoma after a course of 810-nm diode laser TTT delivered using the developed methodology I.V. Tsukanova, Junior Research Associate; S.I. Poliakova, Dr Sc (Med); L.N. Velichko, Cand Sc (Med); A.V. Bogdanova, Cand Sc (Biol) SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) E-mail: inna.sister@gmail.com TO CITE THIS ARTICLE: Tsukanova IV, Poliakova SI, Velichko LN, Bogdanova AV. Changes in immunity characteristics in patients with small (T1) choroidal melanoma after a course of 810-nm diode laser TTT delivered using the developed methodology. J.ophthalmol.(Ukraine).2019;4:28-32. http://doi.org/10.31288/oftalmolzh201942832
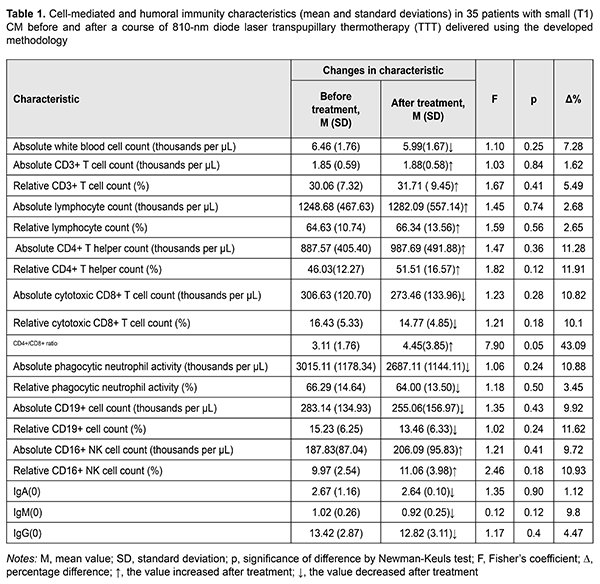
Background: Immune response is an important factor for efficacy of choroidal melanoma (CM) treatment. Adequate uveal melanoma (UM) organ-saving treatment and treatment efficacy monitoring requires determining the interaction pattern between immune and tumor cells. Purpose: To investigate changes in immune characteristics in patients with small T1 CM (measuring ? 3 mm in thickness and ? 12 mm in basal dimension) after a course of 810-nm diode laser transpupillary thermotherapy (TTT) delivered using the developed methodology. Materials and Methods: We determined immune system characteristics in 35 patients (9 men (25.7%) and 26 women (24.3%); mean age, 53.9 (12.1) years) with small (T1) CM before and after a course of 810-nm diode laser TTT delivered using the developed methodology. Results: Some characteristics (absolute white blood cell count, absolute and relative CD8+ T cell counts, absolute and relative phagocytic neutrophil activity, absolute and relative CD19+ B cell counts, IgA, IgM, and IgG) decreased, whereas others (relative white blood cell count, absolute and relative CD3+ T cell counts, absolute and relative CD4+ T helper counts, CD4+/CD8+ T-cell ratio, and absolute and relative CD16+ NK cell counts) increased after treatment. However, these changes were not statistically significant, excepting that relating to CD4+/CD8+ T-cell ratio (F=7.9; p = 0.05). Conclusion: 810-nm diode laser transpupillary thermotherapy (TTT) delivered using the developed methodology results in a shift in specific antitumor immune response patterns with a suppression of cytolytic CD8+ T cell response, which requires administering immunocorrective therapy as early as the initial stage of the disease. Keywords: small T1 choroidal melanoma, transpupillary thermotherapy, cell-mediated and humoral immunity
Introduction In recent years, there have been reports of new ideas of pathogenetic tumor progression mechanisms mediated by the interaction between immune and tumor cells [1-9]. There is a notion that under certain conditions, the immune system does not reject the tumor, but is involved in its development and progression [10]. Uveal melanomas (UM) are highly malignant tumors, and up to 90% of them develop in the choroid. Small choroidal melanomas (CM) (those with a height of up to 3.0-4.0 mm [19-21]) account for 5-21% [11-18] of all CM. According to the 2009 American Joint Committee on Cancer (AJCC)/ Union for International Cancer Control (UICC) staging system, CM measuring ? 3 mm in thickness and ? 12 mm in basal dimension are classified as T1 (small), whereas those measuring 3.1 mm to 6 mm in thickness and > 12 mm in basal dimension are classified as medium [22]. T1 CM is an initial state of the disease, but there is a substantial difference in treatment strategies between patients with small CM and those with medium CM. Adequate and prompt treatment for CM has been found efficient with regard to visual function saving and improved survival prognosis [11-13, 17, 19, 21, 23-29]. Immune response is an important factor for efficacy of CM treatment. Correlations have been reported between cellular and humoral immunity characteristics, on the one hand, and UM patient’s resistance to cancer and disease prognosis, on the other hand [30-33]. Adequate UM organ-saving treatment and treatment efficacy monitoring requires determining the interaction pattern between immune and tumor cells. The purpose of the study was to investigate changes in immune system characteristics in patients with small T1 CM (measuring ? 3 mm in thickness and ? 12 mm in basal dimension) after a course of 810-nm diode laser transpupillary thermotherapy (TTT) delivered using the developed methodology. Materials and Methods We determined immune system characteristics in 35 patients (9 men (25.7%) and 26 women (24.3%); mean age, 53.9 (12.1) years) with small (T1) CM (measuring ? 3 mm in thickness and ? 12 mm in basal dimension) before and after a course of 810-nm diode laser TTT delivered using the developed methodology [34]. Immune system characteristics were investigated using conventional methodologies [35, 36]. Statistical analyses were conducted using Statistica 10 (StatSoft, Tulsa, OK, USA) software. Results and Discussion Table 1 presents the results of the comparison of cellular and humoral immunity characteristics in patients with small (T1) CM before and after a course of TTT delivered using the developed methodology. When TTT exerted its effects on the tumor, CM patient’s body responded by shifts in immunity. Some characteristics (absolute white blood cell count, absolute and relative CD8+ T cell counts, absolute and relative phagocytic neutrophil activity, absolute and relative CD19+ B cell counts, IgA, IgM, and IgG) decreased numerically, whereas others (relative white blood cell count, absolute and relative CD3+ T cell counts, absolute and relative CD4+ T helper counts, CD4+/CD8+ T-cell ratio, and absolute and relative CD16+ NK cell counts) increased. However, these changes were not statistically significant (excepting that relating to CD4+/CD8+ T-cell ratio; F=7.9; p = 0.05). This is possibly due to the short period between “before” and “after” studies, and actual changes have not yet been reflected completely by the immune system. In addition, we have found that, compared to healthy individuals, patients with small (T1) CM had high total numbers of T cells, T helpers and T suppressors [37, 38], which is in disagreement with those of Likhvantseva [9]. At the initial disease stage (when the tumor is small), the immune system is still active, despite the development of tumor process. The curative effect of TTT on small (T1) CM results in increased total numbers of T cells and T helpers and decreased cytotoxic CD8+ T cell counts. A general lymphocytopenia has been reported in patients with UM. In addition, it has been reported that lymphocytopenia became more severe as the tumor and metastases progressed, and was accompanied by variability with regard to T helpers and T suppressors [9]. Given these facts, in patients with UM, immunocorrective therapy should be administered as early as the initial stage of the disease, and the response to this therapy should be monitored over the course of organ-saving treatment.
Conclusion 810-nm diode laser transpupillary thermotherapy (TTT) delivered using the developed methodology results in a shift in specific antitumor immune response patterns with a suppression of cytolytic CD8+ T cell response, which requires administering immunocorrective therapy as early as the initial stage of the disease. References 1.Berezhnaya NM. [Role of immune system cells in tumor microenvironment. II. Interaction of the immune system cells with other microenvironment components]. Onkologiia. 2009;11(2):86–93. Russian. 2.Blokade of PD-L1 (B7-H1) augments human tumor-spescific T cell responses in vitro. Int J Cancer. 2006 Jul 15;119(2):317-27. 3.Mantovani A, Germano G, Marchesi F, et al. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011 Sep;41(9):2522-5. 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. 5.Singh AD, Aronow ME, Sun Y, et al. Chromosome 3 status in uveal melanoma: a comparison of fluorescence in situ hybridization and single-nucleotide polymorphism array. Invest Ophthalmol Vis Sci. 2012 Jun 5;53(7):3331-9. 6.de Waard-Siebinga I, Creyghton WM, Kool J. Effects of interferon alfa and gamma on human uveal melanoma cells in vitro. Br J Ophthalmol. 1995 Sep;79(9):847-55. 7.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008 Aug;9(8):808. 8.de Waard-Siebinga I, Hilders CG, Hansen BE, et al. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1996 Jan;234(1):34-42. 9.Fridman WH, Galon J, Pages F, et al. Prognostic and predictive impact of intra and peritumoral immune infiltrates. Cancer Res. 2011 Sep 1;71(17):5601-5. 10.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow?. Lancet. 2001 Feb 17;357(9255):539-45. 11.Bulgakova ES. [Treatment of small choroidal melanomas with diode laser transpupillary thermotherapy]. [Abstract of Cand Sc (Med) Thesis]. Moscow: Helmholtz Research Institute for Eye Diseases; 2005. 25 p. Russian. 12.Bukhtiiarova NV. [Transpupillary thermotherapy in the multicomponent organ-sparing treatment of choroidal melanoma]. [Abstract of Cand Sc (Med) Thesis]. Cheliabinsk: Ural State Medical Academy of Additional Education; 2006. 22 p. Russian. 13.Volkov VV. [Laser treatment of intraocular melanoma]. Russkii meditsinskii zhurnal. 2001;2(1):3–7. Russian. 14.Mazunin IIu. [New treatment methods in pathologies of the choroid and retina including the use of subthreshold power od fiode infrared laser radiation]. Vestn Oftalmol. 2005 Jan-Feb;121(1):49-54. Review. Russian. 15.Iarovoi AA, Linnik LF, Magaramov DA, et al. [Diode laser transpupillary thermotherapy: the potential for the treatment of small choroidal melanomas]. Russkii meditsinskii zhurnal. 2004;5(2):77–81. Russian. 16.Ushenina LA. [Optimization of laser treatment for early choroidal melanoma]. [Cand Sc (Med) Thesis]. Cheliabinsk: Ural State Medical Academy of Additional Education; 2008. 114 p. Russian. 17.De Potter P, Levecq L. [Transpupillary thermotherapy in the treatment of choroid]. J Fr Ophthalmology. 2001 Nov;24(9):937-43. French. 18.Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123(12):1639-43. 19.De Potter P. [Treatment of intraocular melanoma: new concepts]. Bull Men Acad R Med Belg. 2003;158(1-2):103-11; discussion 111-2. French. 20.Kiratli H, Bilgic S. Peripheral subretinal pigment accumulation following transpupillary thermotherapy for choroidal melanoma. Ophthalmic Surg Lasers Imaging. 2008 Jan-Feb;39(1):60-2. 21.Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmol. – 1995 Sep;102(9):1351-61. 22.Buiko AS, translator. [Choroidal melanoma classification]. Oftalmol Zh. 2010;(6):20-30. Russian. 23.Currie ZI, Rennie IG, Talbot JF. Retinal vascular changes associated with transpupillary thermotherapy for choroidal melanomas. Retina. 2000;20(6):620-6. 24.Damato B, Lecuona K. Conservation of Eyes with Choroidal Melanoma by a Multimodality Approach to Treatment. An audit of 1632 Patients. Ophthalmology. 2004 May;111(5):977-83. 25.Damato B. Developments in the management of uveal melanoma. Clin Experiment Ophthalmol. 2004 Dec;32(6):639-47. 26.Journee–de Korver JG, Oosterhuis JA, De Wolff–Rouendaal D, Kemme H. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Brit J Ophthal. 1997 Mar;81(3):234-9. 27.Shields CL, Shields JA, Cater J. Plaque radiotherapy for uveal melanoma: long–term visual outcome in 1106 consecutive patients. Arch Ophthal. 2000;118:1219–28. 28.Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol. 2002 Jun;13(3):135-41. 29.Shields CL, Shields JA, Cater J, et al. Transpupillary Thermotherapy for Choroidal Melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998 Apr;105(4):581-90. 30.Shields CL, Shields JA, Peres N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002 Feb;109(2):225-34. 31.Gusev AG. [Use of immune modulators in multicomponent treatment of malignant ocular tumors]. Cand Sc (Med) Thesis Abstract. Moscow; 1992. Russian. 32.Likhvantseva VG. [Role of cytokines in the pathogenesis, prognosis and treatment of choroidal melanoma]. Dr Sc (Med) Dissertation Abstract. Moscow; 2001. Russian. 33.Maletskyi AP. [Efficacy of organ-saving treatment of uveal melanoma patients depending on clinical and morphological characteristics of the tumor and body resistance to the tumor]. Dr Sc (Med) Dissertation Abstract. Odesa: Filatov Institute of Eye Disease;2001. 32 p. Ukrainian. 34.Pasyechnikova NV, Naumenko VO, Poliakova SI, Tsukanova IV. [Information Bulletin No. 22 of 25.11.2015, p.145, based on Pat. of Ukraine №102,890 issued 18.05.2015]. [Method for treatment of patients with small (T1) choroidal melanoma]. Owner: State Institution Filatov Institute of Eye Diseases and Tissue Therapy NAMS of Ukraine. Ukrainian. 35.Degtiarenko TV, Bushueva NN, Usov NI. [Methodological guidelines for prompt primary assessment of immune status]. Odessa; 1992. Russian. 36.Gluzman DF, Skliarenko LM, Nadgornaia VA, Kriachok IA. [Immunocytochemistry in tumor diagnosis]. Kyiv: Morion;2003. Russian. 37.Poliakova SI, Velichko LN, Bogdanova AV, Tsukanova IV. [Natural antitumor resistance of the organism condition of patients with uveal melanoma of small sizes]. Oftalmol Zh. 2016;(1):27-38. Russian. 38.De Potter P, Jamart J. Adjuvant Indocyanine Green in Transpupillary Thermotherapy for Choroidal Melanoma. Ophthalmology. 2003 Feb;110(2):406-13; discussion 413-4. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|