J.ophthalmol.(Ukraine).2020;4:77-82.
|
http://doi.org/10.31288/oftalmolzh202047782 Received: 20 April 2020; Published on-line: 27 August 2020 Current aspects of surgical repair for orbital wall fractures: a literature review O.I. Oripov1, E.N. Bilalov1, Sh.A. Boymurodov2 1 Department of Ophthalmology, Tashkent Medical Academy; Tashkent (Uzbekistan) 2 Department of Otorhinolaryngology and Dentistry, Tashkent Medical Academy; Tashkent (Uzbekistan) E-mail: okil.oripov@mail.ru TO CITE THIS ARTICLE: Oripov OI, Bilalov EN, Boymurodov ShA. Current aspects of surgical repair for orbital wall fractures: a literature review. J.ophthalmol.(Ukraine).2020;4:77-82. http://doi.org/10.31288/oftalmolzh202047782 Orbital fractures are an urgent issue in ophthalmological as well as maxillofacial/ plastic surgery practice. The management of orbital fractures is often challenging due to the impact that they can have not only on the cosmetic appearance of the treated area but also on vision. Here we review the major aspects of current reconstructive orbital surgery. This review will primarily focus on the surgical approaches to the fracture site and types of implants available for restoration of the integrity of orbital walls. Their most significant advantages and disadvantages, as well as results of studies on the efficacy of various types of implants, will be reviewed. Keywords: orbit; orbital fractures; reconstructive operations; implants
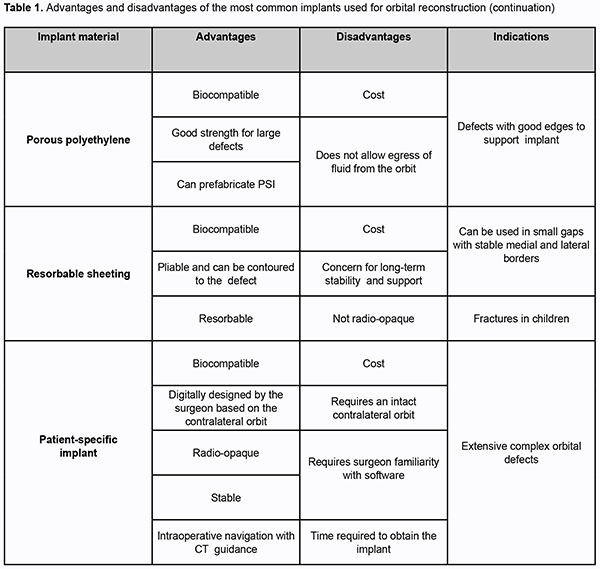
Introduction Orbital fractures are a common trauma, and there are numerous aspects to consider when surgically managing them. They need special attention because surgical management may result in compromise to vision and/or globe position. Males in their first two decades of life are most often affected [1-4]. Orbital fractures in adults are most frequently the result of motor vehicle crash or assault. In children, they are most frequently the result of sports [5]. Orbital fractures are often broadly referred to as “blowout” fractures. However, not all orbital fractures are isolated orbital injuries, they may occur in combination with non-orbital injuries, such as those of the head, neck and/or spine. Many maxillofacial injuries (such as Le Fort type II and III fractures, zygomaticomaxillary complex (ZMC) fractures and nasoorbitoethmoid fractures) involve the orbit [2, 6-8]. Most surgeons [4,9,10] describe the orbital fracture according to the location within the orbit (floor, medial wall, lateral wall, and roof). However, this simplifies the often complex nature of these fractures. A number of classification schemes have been proposed to define isolated, multiwalled, and comminuted orbital fractures, as well as soft tissue displacement [3,4,10]. These schemes improved communication between surgeons, provided guidance on surgical management with regard to indications and timing, and established standards for studies that the orbital fraction patient should undergo. However, management of these injuries has changed little over the years. Advances in maxillofacial/orbital imaging, introduction of intraoperative navigation systems, better evidence-based surgical indications and timing, and improved implant designs have led to a reappraisal of time-honored techniques and guidance [11,12]. Approach The approach to the fracture site depends upon the type of injury, surgeon experience, and available equipment. Subciliary, subtarsal, and transconjunctival incisions are the most commonly utilized. The subciliary approach has been associated with a much higher complication rate, with ectropion resulting in approximately 13% of cases [13,14]. The subtarsal approach is associated with less risk of developing ectropion and if placed appropriately should not result in cicatrization (1%–3%) [15,16]. Most surgeons [7,8,17] prefer the transconjunctival approach to the orbital floor because there is no visible scar and the complication rate is very low – less than 1%. Medial wall fractures are difficult to repair, and there are many surgical approaches to treat them. Some of the most common approaches are the transcutaneous (Lynch incision), transconjunctival inferior fornix, transcaruncular, and endoscopic trans-ethmoidal [6,9,14]. The transcaruncular approach is very popular because it easily combines with the transconjunctival approach [3,4]. Endoscopic approach Interest in the endoscopic approach to the floor and medial wall has increased as surgeons try to avoid eyelid complications and improve visualization of the orbital walls. Cheung et al [18] reviewed nine studies involving 172 patients in which endoscopic approaches were used for orbital wall fractures. No patients had conversion to an open approach and the most common complication was transient cheek numbness. To gain access to the orbital floor, a sublabial approach is utilized to open a window of bone in the anterior wall of the maxillary sinus just below the infraorbital nerve [1,13]. Angled endoscopes are used to visualize the floor defect and the herniated orbital contents. Once the orbital contents are reduced, stable circumferential bony shelves in the floor should be identified, and a flexible implant can be used to close the defect. Some authors [4,18] reported using the anterior maxillary sinus wall bone as an autograft. In medial wall defects, an anterior ethmoidectomy is indicated, which requires some experience with endoscopic sinus surgery [7,14,15]. Image guidance is particularly useful in these cases, although endoscopic approaches can be technically challenging. Even if an eyelid incision is utilized for repair, the endoscope can provide a valuable assessment of soft tissue reduction and implant positioning. In orbital fractures combined with a ZMC or Le Fort fracture where a bony defect in the maxillary sinus already exists, using the endoscope to visualize the orbital floor implant can provide confirmation of proper placement [3,4,7,8,18]. Assessing the state of implants While not a widespread practice, many surgeons [13,19,20] advocate for the use of early postoperative CT imaging to assess implant position. This allows the surgeon to address implant issues early to prevent complications relevant to the globe. Ideally, intraoperative assessment would help solve this issue; this is why its use has become widespread in endoscopic sinus surgery. Preoperative planning using mirror-image overlay of the patient’s normal orbit provides an on-screen guide for placement of the implant. The software to perform mirror-image overlay is not available on all systems. However, even simple image guidance systems can provide important information such as verifying location of a posterior ledge or comparing the slope of proposed implant placement to the contralateral side [11,21,22]. In 113 consecutive cases of complex orbital fractures [11], the use of image guidance was found to significantly decrease the incidence of postoperative diplopia and to significantly reduce to need for revision surgery in fractures that involved multiple orbital walls. Intraoperative imaging has the advantage of showing the actual implant as it is positioned in the orbit, if a radioopaque implant, such as titanium, is used. In recent years, mobile CT scanners have become much less cumbersome and setup and scan times have been reduced to only a few minutes [21,23]. With these units, coronal, sagittal, and even 3D views can be created without a significant increase in radiation exposure. While very thin bone fragments are sometimes difficult to visualize, implant positioning is very clear. In a recent study, Shaye et al [24] found intraoperative CT use to average approximately 14.5 minutes per case, and to have prompted intraoperative revisions in 24% of maxillofacial fracture cases. Selection of implant material Reconstruction of the orbit can be achieved using a wide variety of implants. As with any other type of implant, materials for orbital reconstruction will vary in the specific properties they possess and it will be the surgeon’s assessment of the patient’s fracture, age, location, that will determine the material selection [25,26]. Historically, autografts were the preferred method for orbital reconstruction, while alloplasts have gained popularity with improvement in material engineering and biocompatibility, and now constitute the most widely used implants for orbital reconstruction [9,26-28]. Autogenous bone Although bone has good strength, no sharp edges, can be fixed to adjacent bone and is radiopaque, it can have a variable degree of resorption that can be problematic, and its lack of pliability creates a significant difficulty for adequate molding into complex shapes. The calvarium, iliac crest, nasal, maxillary, and mandibular bone have been used as donor sites, with the first two being the most commonly used [2,4,8,25,26]. Due to the close proximity to the operating field that facilitates harvest and the intrinsic shape of the bone, split calvarial grafts are commonly used. Data accrued in the past 10 years [1,8,13,25,28] have shown that the repair of orbital fractures with calvarium is safe and has an acceptable reduction of enophthalmos and diplopia, but results in less accurate reconstruction of the intrinsic shape of the orbit with less precise recovery of orbital volume. The authors [9,22,28] do not recommend it being used as primary means for reconstruction because of the potential for donor site morbidity, but it could be considered in the setting of fractures in the growing skull. Prospective outcomes for internal orbital reconstruction using a free iliac bone graft were reported by Zunz et al [22] on 24 patients. The technique was considered reliable and with a low rate of enophthalmos and hypophthalmos. There was, however, an 80% rate of bone resorption; therefore, slight overcorrection may be necessary. Autologous cartilage Septal and auricular cartilage has been used for long for reconstruction of orbital defects, but although they are completely biocompatible, they provide limited structural support and are prone to resorption [3,7]. Studies have shown that the harvest technique is simple, and there is minimal to no donor site morbidity, with septal cartilage having better results than conchal cartilage due to the inherent shape of the graft [4,8,28]. Titanium mesh Titanium is highly biocompatible, easily adjusted to architecturally fit simple and complex orbital defects, provides strong support, does not alter its shape or location over time, and it can be easily fixed to adjacent bone. It has well-recognized osseointegration, is easily sterilized, and readily available, although at high cost. Unfortunately, the holes in the plates allow tissue ingrowth that may make removal more difficult, and the cut edges are prone to snaring periorbital soft tissue during placement [7,8,13,25,26]. Studies have reported good outcomes and there has been one report of a surgical site infection requiring implant removal [9,21]. Porous polyethylene This implant material exhibits high biocompatibility, is easily trimmed into any desired shape, can be screw-fixated to bone, and has good strength with good long-term stability. The implant can usually be easily removed if needed, but on occasions, it can break into pieces, making removal more challenging. It has a low-infection rate, and these usually resolve with antibiotics with rare need for implant removal. The material is readily available, although at a high cost. There is no donor site morbidity or costs associated with increased operative time for implant harvest [1,6,26,27]. However, it is not radiopaque. There are titanium-reinforced porous polyethylene sheets, which combine the favorable properties of both implants. The titanium allows for easier fixation into bone and precise manipulation of the implant to fit complex orbital defects, and it makes the implant radiologically visible [7-9,25]. Resorbable sheeting Sheets made of poly-l/d-lactide, polyglactin, and polydioxanone have been commercially made from resorbable materials for orbital reconstruction. These are pliable and can be contoured to the orbital defect and have a very low infection rate [1,9,25,28]. Some authors [4,21,27] suggest that if the implant is placed under periosteum, the surrounding tissues will create a fibrous scar preventing prolapse of tissues into the maxillary sinus after resorption, while others raise concerns for loss of long-term structural support and recommend its use for defects <2.5 cm2. Further studies looking at long-term outcomes are needed to assess the long-term stability of the reconstruction. Patient-specific implants Using preoperative CT data, a construct can be specifically designed to mirror the non-affected orbit, thus creating a patient-specific implant (PSI). Titanium, polyetheretherketone, and glass-bioceramic have been used to manufacture PSI. These anatomically ideal models are intended to reduce the need for intraoperative manipulation, thus reducing operative time with more accurate reconstruction [2,6,8,11]. Initial studies are promising demonstrating accurate fitting on all implants, no persistent postoperative visual impairments and no patient-reported sensation of foreign body [11,12]. Unfortunately, there are still some limitations with the software from loss of data of thin bone altering the shape of the implant, as well as the risk of incorporating impurities into the implant resulting in rejection [19,24]. Advantages and disadvantages of the most common implants used for orbital reconstruction are presented in Table 1 (adapted from Boyette et al [9]). Complications The most common postoperative complications are diplopia, enophthalmos, and ectropion [1,3,13]. The incidence of the most worrisome complication, vision loss after surgery, has been reported as between 0% and 0.4% [4,7]. Transient diplopia after surgery is common and will typically improve or resolve in a few weeks. However, the reported incidence of persistent diplopia ranges from 8% to 42% [3,8,21]. Postoperative diplopia has been found to be more likely in older patients and those whose fracture repair was delayed [1,7]. Some consideration should be given to earlier repair (immediate or within a few days) in cases where periorbital tissues may be entrapped and damaged. The reported incidence of enophthalmos following surgical repair ranges from 7% to 27% [4,8]. Fat atrophy is speculated as a common reason for this finding, but it may be due to inadequate reconstruction of the orbital cone. Fortunately, this can be corrected with secondary implant augmentation (additional plates or replacement) approximately 3 months after the initial surgery. Avoiding the use of subciliary incisions may decrease the incidence of postsurgical ectropion [16].
Conclusion It should be our goal to reduce the occurrence of the aforementioned complications – many of which are related to inadequate intraoperative assessment and implant placement. Less traumatic surgery with more accurate anatomic reconstruction is needed. Further studies are needed to determine which cases are best approached endoscopically. Currently, several centers are using preoperative CT imaging to quickly create customized 3D implants for each individual defect. Intraoperative navigation can then be used to precisely place the implant according to the preoperative planning based upon the normal orbit. Rapid, cost-effective production of such implants is the next logical step, and developments in point-of-care 3D printing are promising. Customized orbital implants and intraoperative CT imaging used with image guidance technology should improve accuracy of implant placement and lead to better patient outcomes.
References 1.Kataev MG, Eolchiian SA, Tishkova AP. [Orbital fractures: Diagnosis and treatment policy]. Vestn Oftalmol. Jan-Feb 2006;122(1):26-32. Russian. 2.Lutsevich EE, Al'khumidi K. [Modern aspects of diagnostics and treatment of orbit fractures]. Vestn Oftalmol. 2013;6:89-94. Russian. 3.Nikolaenko VP, Astakhov YS. Orbital fractures: a physician's manual. Berlin: Springer Berlin Heidelberg; 2015. 4.Ellis E 3rd. Orbital trauma. Oral Maxillofac Surg Clin North Am. 2012;24(4):629–48. 5.Oppenheimer AJ, Monson LA, Buchman SR. Pediatric orbital fractures. Craniomaxillofac Trauma Reconstr. 2013;6(1):9–20. 6.Bakushev AP, Sivolapov KA. [Surgical treatment of patients with isolated fractures of the orbit walls]. Ophthalmology in Russia. 2015;12(3):48-53. Russian. 7.Cole P, Kaufman Y, Hollier L. Principles of facial trauma: orbital fracture management. J Craniofac Surg. 2009;20(1):101–4. 8.Gart MS, Gosain AK. Evidence-based medicine: orbital floor fractures. Plast Reconstr Surg. 2014;134(6):1345–55. 9.Boyette RJ., Pemberton J.D., Bonilla-Velez J. Management of orbital fractures: challenges and solutions. Clinical Ophthalmology. 2015;9:2127–2137. 10.Kunz C, Audige L, Cornelius CP, Buitrago-Tellez CH, Rudderman R, Prein J. The comprehensive AOCMF classification system: orbital fractures – level 3 tutorial. Craniomaxillofac Trauma Reconstr. 2014;7(Suppl 1):S092–S102. 11.Rana M, Chui CH, Wagner M, Zimmerer R, Rana M, Gellrich NC. Increasing the accuracy of orbital reconstruction with selective lasermelted patient-specific implants combined with intraoperative navigation. J Oral Maxillofac Surg. 2015;73(6):1113–8. 12.Raschke G, Rieger U, Bader RD, Schaefer O, Guentsch A, Schultze-Mosgau S. Outcomes analysis of eyelid deformities using photograph-assisted standardized anthropometry in 311 patients after orbital fracture treatment. J Trauma Acute Care Surg. 2012;73(5):1319–25. 13.Grusha OV, Grusha IaO. [Five Hundred Orbital Plastic Repairs: Analysis of Complications]. Vestn Oftalmol. 2006;1:22-4. Russian. 14.Eolchiian SA, Kataev MG, Karnaukhova AV. [Repairing functional and cosmetic abnormalities in the surgical management of traumatic defects and malformations of the orbit and periorbital region]. Esteticheskaia meditsina. 2012;11:4:3-14. Russian. 15.Malanchuk VA, Astapenko OO, Chepurnyi IuV, Logvinenko IP. [Potential for restoring the integrity of the orbit and ocular adnexa in patients with midface fractures]. Ukrainskyi Medychnyi Chasopys. 2012;1(87):124-6. Russian. 16.Feldman EM, Bruner TW, Sharabi SE, Koshy JC, Hollier LH Jr. The subtarsal incision: where should it be placed? J Oral Maxillofac Surg. 2011;69(9):2419–23. 17.Kothari NA, Avashia YJ, Lemelman BT, Mir HS, Thaller SR. Incisions for orbital floor exploration. J Craniofac Surg. 2012;23(7 Suppl 1):1985–9. 18.Cheung K, Voineskos SH, Avram R, Sommer DD. A systematic review of the endoscopic management of orbital floor fractures. JAMA Facial Plast Surg. 2013;15(2):126–30. 19.Kozakiewicz M. Computer-aided orbital wall defects treatment by individual design ultrahigh molecular weight polyethylene implants. J Craniomaxillofac Surg. 2014;42(4):283–9. 20.Liao JC, Elmalem VI, Wells TS, Harris GJ. Surgical timing and postoperative ocular motility in type B orbital blowout fractures. Ophthal Plast Reconstr Surg. 2015;31(1):29–33. 21.Strong EB. Orbital fractures: pathophysiology and implant materials for orbital reconstruction. Facial Plast Surg. 2014;30(5):509–17. 22.Zunz E, Blanc O, Leibovitch I. Traumatic orbital floor fractures: repair with autogenous bone grafts in a tertiary trauma center. J Oral Maxillofac Surg. 2012;70(3):584–92. 23.Gander T, Essig H, Metzler P, et al. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J Craniomaxillofac Surg. 2015;43(1):126–30. 24.Shaye DA, Tollefson TT, Strong EB. Use of intraoperative computed tomography for maxillofacial reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):113–9. 25.Grusha IaO, Fedorov AA, Bakaeva TV. [Comparative experimental study of current implant materials used in orbital surgery]. Vestn Oftalmol. 2012;2: 27—33. Russian. 26.Davydov DV. [Characteristics of materials used in surgical correction of orbit walls]. Annaly plasticheskoy, rekonstruktivnoy i esteticheskoy khirurgii. 2009;3:52—8. Russian. 27.Alonso-Rodriguez E, Cebrian JL, Nieto MJ, Del Castillo JL, HernandezGodoy J, Burgueno M. Polyetheretherketone custom-made implants for craniofacial defects: report of 14 cases and review of the literature. J Craniomaxillofac Surg. 2015;43(7):1232–8. 28.Gunarajah DR, Samman N. Biomaterials for repair of orbital floor blowout fractures: a systematic review. J Oral Maxillofac Surg. 2013;71(3):550–570. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|