J.ophthalmol.(Ukraine).2020;4:33-37.
|
http://doi.org/10.31288/oftalmolzh202043337 Received: 14 June 2020; Published on-line: 27 August 2020 Brain potential response to activation procedures in children with refractive amblyopia I.M. Boichuk, Badri Wael SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: iryna.ods@gmail.com TO CITE THIS ARTICLE: Boichuk IM, Badri Wael. Brain potential response to activation procedures in children with refractive amblyopia. J.ophthalmol.(Ukraine).2020;4:33-37. http://doi.org/10.31288/oftalmolzh202043337 Purpose: To identify the features of cortical potential response to activation procedures in patients with mild and moderate refractive amblyopia. Material and Methods: We examined electroencephalography (EEG) response to activation procedures (specifically, eye opening and photic stimulation) in 22 pediatric amblyopes and 15 healthy children. All subjects underwent an eye examination, including visual acuity measurement, ophthalmoscopy, and refractometry. In addition, binocular function was assessed using the Worth 4-dot test and synoptophore. An EEG response to alternating unilateral eye opening was used as an indicator of the integrity of the prechiasmal pathway. A response to 10-Hz rhythmic photic stimulation (rhythmic driving response, RDR) was used as a measure of maturity of cortical neurons. The performance of the thalamocortical relay was assessed through the RDR to unilateral and bilateral photic stimulation with the eyes closed to exclude the effect of reticular formation. In addition, eyes closed EEG synchronization was assessed at symmetric EEG derivations to detect the absence of connection through the corpus callosum. Results: In the total sample of children with mild and moderate refractive amblyopia, there was an increased rate of a weak desynchronization response to bilateral eye opening and to unilateral eye opening in the hemisphere ipsilateral to the amblyopic eye. Particularly, a weak response was exhibited by all patients without binocular vision. In addition, we found the absence of slow waves in both hemispheres during rhythmic driving response to binocular photic stimulation and during response to bilateral eye opening. Conclusion: Findings of this study (1) evidence immaturity of cortical and midbrain neurons and (2) confirm the integrity of the major retinal cortical pathway and retinal thalamic cortical pathway and their interhemispheric connection in children with mild and moderate refractive amblyopia. Keywords: refractive amblyopia, brain potential, electroencephalography, EEG
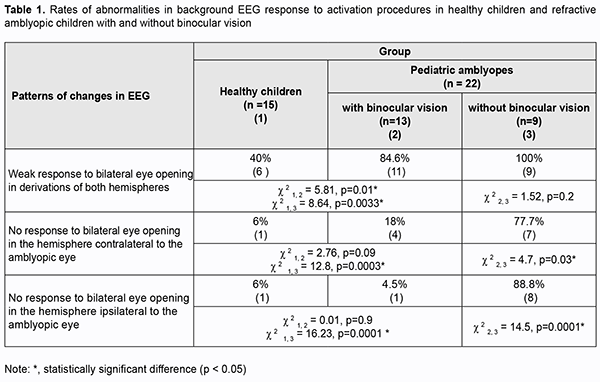
Introduction Amblyopia is a common cause of visual loss in children, accounting for 4-6% of cases of pediatric eye disorders. Findings of recent animal and clinical studies on the pathogenesis of amblyopia allowed concluding that amblyogenic factors prevent a healthy visual pathway from forming between the eye and the brain during the critical period of neurodevelopment [1]. Amblyopia is characterized by histopathological changes in the visual cortex and lateral geniculate nucleus, and the severity of these changes depends on the type of amblyopia. No retinal morphological changes have been found in amblyopic eyes [2, 3]. However, functional changes have been reported in the retinal receptive fields in amblyopic eyes [4-8], and these fields are implicated in contrast sensitivity and pattern vision. Crawford and colleagues [3] reported that the major abnormality in this disease lies in loss of binocular cortical neurons that respond to stimulation of either eye. Studies on deprivation found that deprivation affects not so much retinal morphology but rather retinal neurotransmitters, contributing to decreased dopamine and tyrosine hydroxylase synthesis and increased vasoactive intestinal peptide levels, as well as arborization of retinal neurons. Eccentric fixation may occur in amblyopia. Analysis of electroencephalogram (EEG) visual-evoked potentials (VEPs) is important for assessing the visual pathway integrity in general. EEG with VEP recording is used for clinical studies in neonates and uncooperative children, as well as for animal studies [9-11]. VEP represents the response of large populations of visual cortex neurons to synchronous stream of pulses induced by an afferent stimulus. Since the VEP reflects the activity of the sensory visual pathways from the retina to the visual cortex, pathology in any portion of the visual pathway causes changes in VEP [12]. Therefore, by comparing the EEGs recorded before and during activation procedures in amblyopes and healthy individuals, one may not only assess the state of the central visual system in general, but also determine rather accurately whether the central nervous system injury is functional or structural, and whether the injury is located in cortical areas, subcortical areas of the brain stem, or neuronal structures of the brain stem. Because functional abnormalities of the cortical and subcortical visual system are involved in the etiopathogenesis of amblyopia, EEG studies in amblyopes have been interesting for ophthalmologists for decades. In the 1970s and 1980s, studies by Soviet ophthalmologists [13, 14] investigated EEG alpha rhythm patterns in strabismic patients and found an alpha interhemispheric asymmetry in these patients. In a study by Shibinskaia (1975) [13], the EEG showed signs of brainstem abnormalities and abnormalities of the inferior portion of the midbrain in 17 of the 23 strabismic children. Dobromyslov [15] found interhemispheric asymmetry in alpha amplitude in children with strabismic amblyopia and strabismus (without amblyopia). No occipital alpha rhythm was found in patients with visual acuity of less than 0.1. In addition, that study found no difference in EEG parameters between patients with alternating strabismus and those with paralytic strabismus. In a study by Avetisov and colleagues [16], 82.2% of cases with accommodative strabismus showed pathological EEG patterns. They found changes in the alpha rhythm characteristics and occurrence of pathologic delta and theta waves in 91 of 110 patients, which indicated damage to the brainstem, basal and pontine structures. They, however, did not mention, whether there were amblyopes among the study objects. Subsequent studies (those by Shamshinova and colleagues [14], Zislina [17, 18], Dubovskaia [19], etc.) focused on differences in brain potential response (particularly, primary visual cortex (Brodmann area 17) response) to flash or pattern reversal stimuli among healthy individuals, amblyopes and patients with retinal and/or optic nerve disorders. These studies determined configuration parameters, latency estimates, and differences in evoked potential amplitude, and identified the prognostic value of these characteristics. Thus, configuration of pattern-evoked potentials (PEP) became simpler, amplitude of the major PEP peaks decreased, and latency of the major PEP peaks increased with an increase in severity of amblyopia. Dubovskaia (1997) [19] found an abnormal PEP configuration, a decrease in amplitude (particularly, a 5-fold decrease in P100 amplitude) and a more than 10 ?s increase in P100 latency in 50% of children with failed treatment for unilateral amblyopia. She believes that the above changes in PEP parameters indicate an organic injury of the visual system. Shamshinova and colleagues (2002) and Zislina (1991, 1993) [14, 20, 17, 18, 13] used pattern VEP topographic mapping and analyzed the VEP that were extracted from the occipital EEG of patients with refractive and strabismic amblyopia. They demonstrated normal VEP composition and distinct difference in configuration between flash- and pattern reversal evoked-VEP for these patients. Those authors believe that (1) the above indicates maintained reactivity of the system of intracortical connections, the system that provides for image pattern analysis, and (2) the identified features of the VEP of patients with amblyopia of obscure origin are likely to reflect immaturity of this system. They also noted decreased excitability of cortical neurons and slow data transmission to cortical visual centers. A difference in amplitudes reflects a decreased percentage of responded cortical neurons. To the best of our knowledge, there have been limited foreign EEG studies in amblyopia reporting evidence of immature visual system like long VEP latency and difference in amplitude for occipital VEP (particularly, small occipital P100 amplitude) of the amblyopic eye [6, 7, 9, 21]. Orel-Bixler [10] demonstrated reduced VEP amplitude after high spatial frequency black and white gratings were presented to the amblyopic child’s eye. Those authors, however, did not mention the type of amblyopia. Generally, we can clearly state that EEG features of various types of amblyopia have been poorly defined. The above authors considered responses in the occipital derivations, but not other derivations in amblyopia. EEG signs of impaired subcortical structures associated with the visual motor apparatus have not been studied in this disease. However, the motor components of vision are as much important for visual perception as the sensory components. The purpose of this study was to identify the features of cortical potential response to activation procedures in patients with mild and moderate refractive amblyopia. Material and methods Twenty-two 5-year- to 8-year-old children with refractive amblyopia underwent EEG recording. Each amblyopic child had a BCVA of between 0.3 and 0.6; an interocular difference in BCVA of 0.2 or less; hypermetropia of 4 to 8 D; astigmatism of 2.0 D or less; central fixation and normal convergence. Thirteen amblyopic children (60%) had binocular vision as assessed by the Worth test, and all exhibited normal fusional amplitude as assessed by the synoptophore. The control group comprised of 15 healthy age-matched children with a BCVA of between 0.8 and 1.0, no deviation, hypermetropia of 0.25 to 0.75 D, and normal binocular functions. EEG was performed using an 8-channel EEG machine (Medicor EEY8S) and a 16-channel computer QUATTOR system employing 10–20 system of electrode placement for 8 children and 29 children, respectively. Rhythms of the background EEG, interhemispheric asymmetry and alpha, theta and delta wave characteristics were assessed. Brain potentials were recorded using standard procedures to activate deep brain structures. EEG response to bilateral eyes opening through the alpha-rhythm depression reaction. In addition, activating function of the rostral reticular formation was assessed by the hyperventilation EEG through increased theta and delta-wave index in the frontal and anterior parietal derivations [22]. An EEG response to alternating unilateral eye opening was used as an indicator of the integrity of the prechiasmal pathway. A response to 10-Hz rhythmic photic stimulation (the rhythmic driving response, RDR) was used as a measure of maturity of cortical neurons. The performance of the thalamocortical relay was assessed through the rhythmic driving response to unilateral and bilateral photic stimulation with the eyes closed to exclude the effect of reticular formation. In addition, eyes closed EEG synchronization was assessed at symmetric EEG derivations (Fp2 - F4 ? Fp1 - F3; F4 - C4 ? F3 - C3; C4 - P4 ? C3 - P3; P4 - O2 ? P3 - O1) to detect the absence of connection through the corpus callosum [22, 23]. Statistical analyses were conducted using Statistica 8 (StatSoft, Tulsa, OK, USA) software. The chi?squared test was used to identify frequency differences in amblyopia groups with and without binocular vision. Results Among the control children, 65.0% exhibited a well-organized EEG (as per the classification by Zhirmunskaia [24]). In addition, regional differences were preserved and predominant in 80.0% of the controls. The interhemispheric asymmetry of 10% or less was found in 60.0% of the control children. The EEG alpha rhythm was poorly modulated, predominant in the occipital derivations, and was of 7-8 Hz and 20-40 ?V amplitude in 68.8% of the control children. Slow activity in the form of theta and delta waves (25-30 ?V) was more common in the occipital derivations in 15.0% of healthy children. Unilateral or bilateral eye opening-closing was found to block alpha rhythm. The rhythmic driving response was moderate for both hemispheres, and the amplitude difference between hemispheres was not significant. Photic stimulation resulted in symmetric waveforms in transverse derivations of the EEG, with the number of slow oscillations corresponding to the background activity. Hyperventilation resulted in synchronization of alpha rhythm in 60% of healthy children, and in occurrence of slow theta and delta waves in frontal derivations not exceeding 20% of recording time in 40% of healthy children. As per the recommendations by Gnezditskii [23] and Zenkov [22], these values were considered to be normal for healthy children aged 5 to 8 years. The parameters of the background EEG in children with refractive myopia generally corresponded to the above age-matched reference values. The interhemispheric asymmetry of 10% or less was found in 68.0%, and increased alpha fronto-occipital coherence was seen in 65.0 % of children with refractive myopia. In addition, the amount of theta and delta activity was somewhat increased, with theta index and delta index being of 27.5% and 30.0%, respectively, but the difference from a healthy age-matched reference population was not significant (p > 0.05). Unilateral or bilateral eye opening-closing was found to block alpha rhythm in all children with refractive amblyopia. No pathological activity was found in the EEG response to hyperventilation. However, after unilateral eye opening-closing, children with refractive amblyopia exhibited significantly weaker alpha rhythm attenuation in the hemisphere ipsilateral to the eye with lower vision, compared to healthy controls, which was demonstrated by a subtle change in alpha rhythm amplitude in occipital derivations in 52% of children with amblyopia. The rhythmic driving response was moderate in all amblyopic children. There was no change in interhemispheric alpha coherence in symmetric derivations during photic stimulation. The normal alpha rhythm desynchronization response to exposure to light (which is expressed through reduced rhythm amplitude and increased rhythm frequency) was noted in practically all amblyopic patients. However, compared to healthy controls, this response was lower in 52% of total patients. In addition, the response was low in all subjects without binocular vision (Table 1). There were differences in the response to eye opening in the hemispheres ipsilateral and contralateral to the amblyopic eye between amblyopic children with and without binocular vision. No response to bilateral eye opening in occipital derivations of the hemispheres contralateral and ipsilateral to the amblyopic eye was much more common in the latter patients (77.7% and 88.8%, respectively) than in the former patients as well as healthy controls (p < 0.05) (Table 1).
More generally, this EEG study revealed a weakened normal alpha rhythm desynchronization response (alpha rhythm blocking or attenuation) in amblyopic children compared to healthy controls, indicating loss of input to the visual cortical projection areas, especially in amblyopic children without binocular vision (100%). Because it is the brainstem reticular formation that enables desynchronization (or activation) of the visual cortex, an absence of this desynchronization is caused by dysfunction of this formation, which might also indicate the presence of oculomotor impairment in amblyopic patients. Discussion Percentages of alpha, beta, delta and theta rhythms in the EEG record, frequencies and amplitudes of these waves, and the level of alpha-rhythm suppression are essential for characterizing the function of the visual system [17, 20]. Zenkov [22] reported that insufficient activation of the alpha rhythm during eye opening indicates a reduced activating capacity of the reticular formations in rostral (superior) and pontine (middle) brainstem as well as immaturity of the midbrain structures. The response to rhythmic photic stimulation depends on the state of the thalamocortical relay and mesencephalic reticular formation [13, 22, 23]. Children with refractive amblyopia exhibited a satisfactory response to rhythmic photic stimulation. Therefore, the results of our EEG study in children with mild and moderate refractive amblyopia indicate immaturity of cortical and midbrain neurons as well as cortical-subcortical relationships in these children, as evidenced by increased rate of a weak desynchronization response to bilateral eye opening and to unilateral eye opening in the hemisphere ipsilateral to the amblyopic eye. We found the absence of slow waves in both hemispheres during rhythmic driving response to binocular photic stimulation and during response to bilateral eye opening, which confirms the integrity of the major retinal cortical pathway and retinal thalamic cortical pathway and their interhemispheric connection in children with mild and moderate refractive amblyopia. In addition, we found loss of input to the visual cortical projection areas in pediatric amblyopes without binocular vision, which was evidenced by a weakened response to bilateral eye opening in both hemispheres for all relevant cases. References 1.Demer JL, von Noorden GK, Volkow ND. Imaging of cerebral blood flow and metabolism in amblyopia by positron emission tomography. Am J Ophthalmol. Am J Ophthalmol. 1988 Apr 15;105(4):337-47. 2.Hubel DH, Wiesel TN. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41-2. 3.Crawford MLJ, von Noorden GK, Meharg LS, et al. Binocular neurons and binocular function in monkeys and children. Invest Ophthalmol Vis Sci. 1983. 1983 Apr;24(4):491-5. 4.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215-43. 5.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377-409. 6.Ikeda H. Is amblyopia a peripheral defect? Trans Ophthalmol Soc UK. 1979;99(3):347-52. 7.Ikeda H. Visual acuity, its development and amblyopia. J R Soc Med. 1980 Aug;73(8):546-55. 8.Ibatulin RA. [Visual functions in amblyopia as assessed by psychophysical and electrophysiological studies]. Thesis of dissertation for the degree of Dr Sc (Med). Moscow: Helmholtz Research Institute of Eye Diseases; 1998. Russian. 9.Norcia AM, Tyier C. Spatial frequency sweep VEP: visual acuity during the first year of life. Vision Res. 1985;25(10):1399-408. 10.Orel-Bixler DA. Subjective and VEP measures of acuity in normal and amblyopic adults and children. University of California, Berkeley, 1989. 11.Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 01 Mar 2003, 43(6):729-738 12.DOI: 10.1016/s0042-6989(02)00684-3. 13.Peper E, Mulholland T. Methodological and theoretical problems in the voluntary control of EEG occipital alpha by the subject. Kybernetik. 1970. 7:10-3. 14.Shibinskaia NI. [Study of bioelectric activity of the cerebral cortex in dysbinocular amblyopia according to electroencephalographic data]. Oftalmol Zh. 1975;30(6):467-70. 15.Shamshinova AM, Iakovlev AA, Romanova EV. [Clinical physiology of vision]. Moscow: MBN; 2002. Russian. 16.Dobromyslov AN. [On the conditional reflex-associated nature of binocular vision and concomitant strabismus]. Vestn Oftalmol. 1963;3:160-5. Russian. 17.Avetisov SE. [Strabismic amblyopia and its management]. Moscow: Meditsina; 1968. p.5-38, 207. Russian. 18.Zislina NN, Sorokina RS. [The effect of functional and organic disorders in the visual system on the amplitude-temporal characteristics of evoked potentials]. Fiziol Cheloveka. 1991 May-Jun;17(3):27-33. 19.Zislina NN, Shamshinova AM. [Physiological basics and potential for application of visual evoked potentials in differential diagnosis of eye disease]. In: [Clinical physiology of vision]. Moscow: Rusomed; 1993. p. 146-57. Russian. 20.Dubovskaia LA. [Pathogenesis targeted therapeutic approaches in children with amblyopia and partial optic nerve atrophy]. Thesis of dissertation for the degree of Dr Sc (Med). Moscow: Helmholtz Research Institute of Eye Diseases; 1977. Russian. 21.Zinchenko VP, Vdovina LI, Gordon VM. [Study on the functional structure of combinatory problem solving]. In: [Motor components of vision]. Moscow: Nauka; 1975. Russian. 22.Hou C, Good WV, Norcia AM. Norcia. Detection of Amblyopia Using Sweep VEP Vernier and Grating Acuity. Invest Ophthalmol Vis Sci. 2018 Mar 1;59(3):1435-1442. 23.Zenkov LR. [Electroencephalography (with Elements of Epileptology)]. Taganrog: TGRU; 1996. Russian. 24.Gnezditskii VV, Shamshinova AM. [Clinical experience of the use of evoked potentials]. Moscow: MBN; 2001. Russian. 25.Zhirmunskaia EA, Maiorchik VE, Ivanitskii AM. [Terminology manual (glossary of EEG terms)]. Vol. 4. Moscow: Meditsina; 1978. p. 936-54. Russian. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|